An example of an alkane halogenation reaction is. Alkanes are simplest organic compounds that consist of single bonded carbon and hydrogen atoms with the general formula cnh2n+2. The hydrocarbon meaning is that these compounds exclusively consist of hydrogen and carbon atoms.
Alkane Model from Organic Model Set 62009
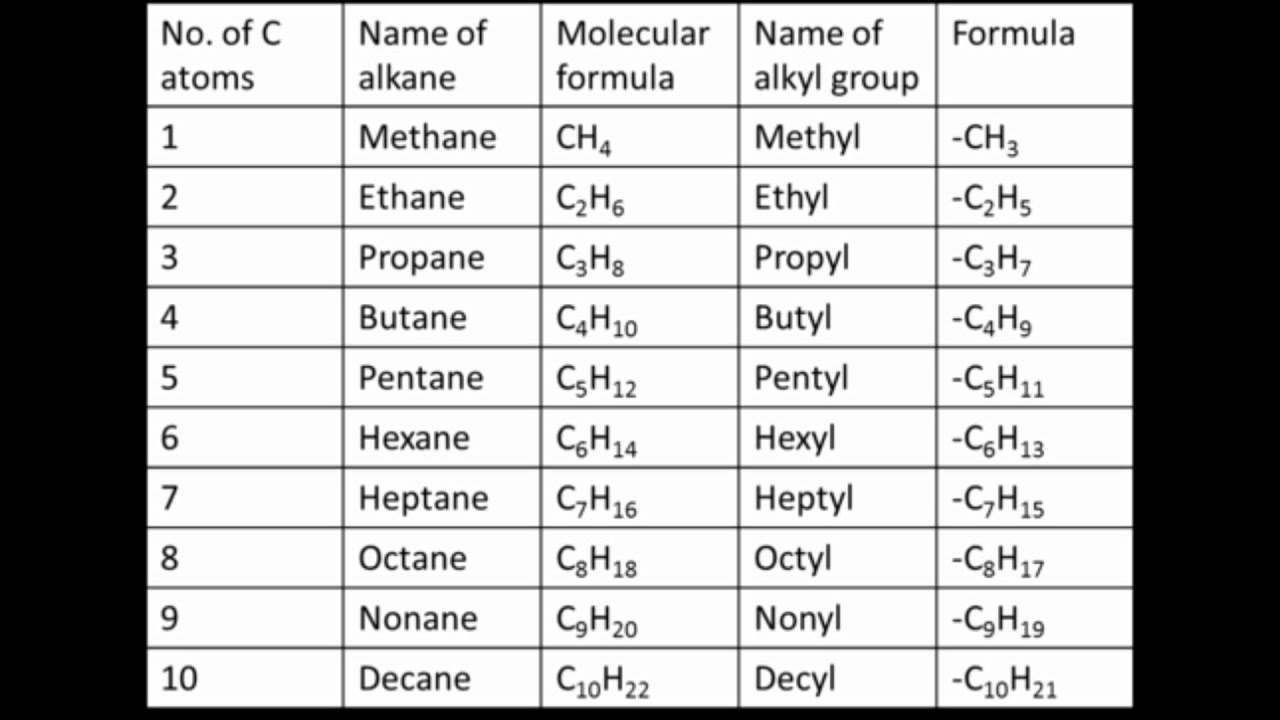
For example, methane is ch 4 and ethane is c 2 h 6.
The simplest alkane is composed of a single carbon bonded to four hydrogens, methane {eq}ch_4 {/eq}.
Almost an unlimited number of derivatives can be made from the alkanes since any hydrogen can be substituted by an alkyl group, a halide, etc. Introduction to alkane alkanes are saturated hydrocarbon consist of carbon and hydrogen only without any functional group. The meaning of alkane is any of numerous saturated hydrocarbons; Alkanes are solid, liquid or gas at room temperature depending on the size of their molecules.to learn detailed structures, formulas, and physical properties of alkanes with faqs and videos, visit byju’s for more information.
Each alkane name is built from a prefix (first part) and a suffix (ending).
Let us learn in detail about alkanes and their physical properties below. If the substituent is an alkyl group, then the derivative will have the same empirical formula as a larger alkane, so the empirical formula for an organic compound is insufficient to identify it. Damit sind sie eine untergruppe der. Die allgemeine formel der alkane lautet c n h 2n+2 mit n = anzahl der kohlenstoffatome.
Two general types of monoalkenes are distinguished:
There is a chemical bond between two atoms or groups of atoms when the forces acting between them are strong enough to lead to the formation of an aggregate with sufficient. By saturated hydrocarbons, it means alkanes have single hydrogen and carbon atoms in their chemical formula. Alkane halogenation is an example of a substitution reaction a type of reaction that often occurs in organic chemistry. The general formula means that the number of hydrogen atoms.
Alkane (ăl`kān), any of a group of aliphatic hydrocarbons whose molecules contain only single bonds (see chemical bond chemical bond, mechanism whereby atoms combine to form molecules.
Formula of alkane is c n h 2n+2. Because this compound only has a single covalent bond, therefore, its chemical structure is (image to be added soon) long chain alkane molecules It was discovered that crudes with the same. Methane, propane, ethane, and butane are four alkanes.
The carbon skeleton is how many carbons are linked to each other.
By applying our expertise in technology and data science, we work with our partners and customers in systematically identifying, measuring and eliminating sources of fugitive methane emissions across the oil and gas industry in an effort to fulfill our role in. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds. Linear alkanes branched alkanes cyclic alkanes 1. Each carbon atom participates in 4 chemical bonds.
Halogenation of an alkane produces a hydrocarbon derivative in which one or more halogen atoms have been substituted for hydrogen atoms.
Methane (ch4), ethane (c2h6), propane (c3h8) etc. Als alkane (grenzkohlenwasserstoffe, früher paraffine) bezeichnet man in der organischen chemie die stoffgruppe der gesättigten, acyclischen kohlenwasserstoffe.das heißt, ihre vertreter bestehen nur aus den beiden elementen kohlenstoff (c) und wasserstoff (h), weisen nur einfachbindungen und keine kohlenstoffringe auf. The name methane is composed of two parts: Simple alkane methane contains one carbon atom and four hydrogen atoms, and ch 4 is its molecular formula.
In an alkane is double the number of carbon atoms, plus two.
Classification of alkane there are three types of alkanes: Every hydrogen is joined to a.