In this video we differentiate between redox reactions vs reactions that add oxygen/hydrogen without a net reduction. Oxidation and reduction of alkenes and alkynes. The steps involved in reduction of an alkyne to a trans alkene in order.
PPT OXIDATION AND REDUCTION OF ALKENES PowerPoint
Oxidation can be defined as the addition of oxygen to a molecule or the removal of hydrogen from a molecule.
406 11 alkenes and alkynes ii.
Reduction of alkynes is a useful method for the stereoselective synthesis of disubstituted alkenes. Diimide reduction is a mild and neutral alternative. 73 % • use of an achiral salen complex in conjunction with a second ligand gives good (and cheaper, control what have we learnt? This means that alkynes can be reduced by the addition of one or two equivalents of h 2, to alkenes and alkanes respectively:
Alkenes undergo a number of reactions in which the c=c double bond is oxidized.
Alkenes to alkanes alkenes are transformed to alkanes through the treatment with hydrogen over a finely divided metal catalyst like palladium, nickel, or platinum shown in figure. 1.) an electron adds to the triple bond to form a radical anion. 10.7 oxidation reactions of alkenes. In a hydrogenation reaction, two hydrogen atoms are added across the double bond of an alkene, resulting in a saturated alkane.
This video takes you through the various oxidation and reduction reactions you've covered back in alkene and alkyne reactions.
The product of this reaction is an alkane. 2.) the solvent nh₃ supplies an h⁺ ion, forming a radical intermedatie. One of these preparation methods of alkanes was given by wurtz which is called wurtz reaction. Which of the following options correctly describe the addition of h2 to an alkene?
When alkyl halide reacts with metallic sodium in presence of dry ether, it forms alkane with double the number of carbon atoms present in the alkyl halide of reactants.
Intrinsically, hydrogenation of alkenes has a large energy barrier, making the reaction unfavorable at room temperature. Reduction, oxidation and homologation of alkenes. Hydrogenation of a double bond is a thermodynamically favorable reaction because it forms a more stable (lower energy) product. In a hydrogenation reaction, two hydrogen atoms are added across the double bond of an alkene, resulting in a saturated alkane.
Acidity of alkynes compound would be oxidized if the reverse change took place:
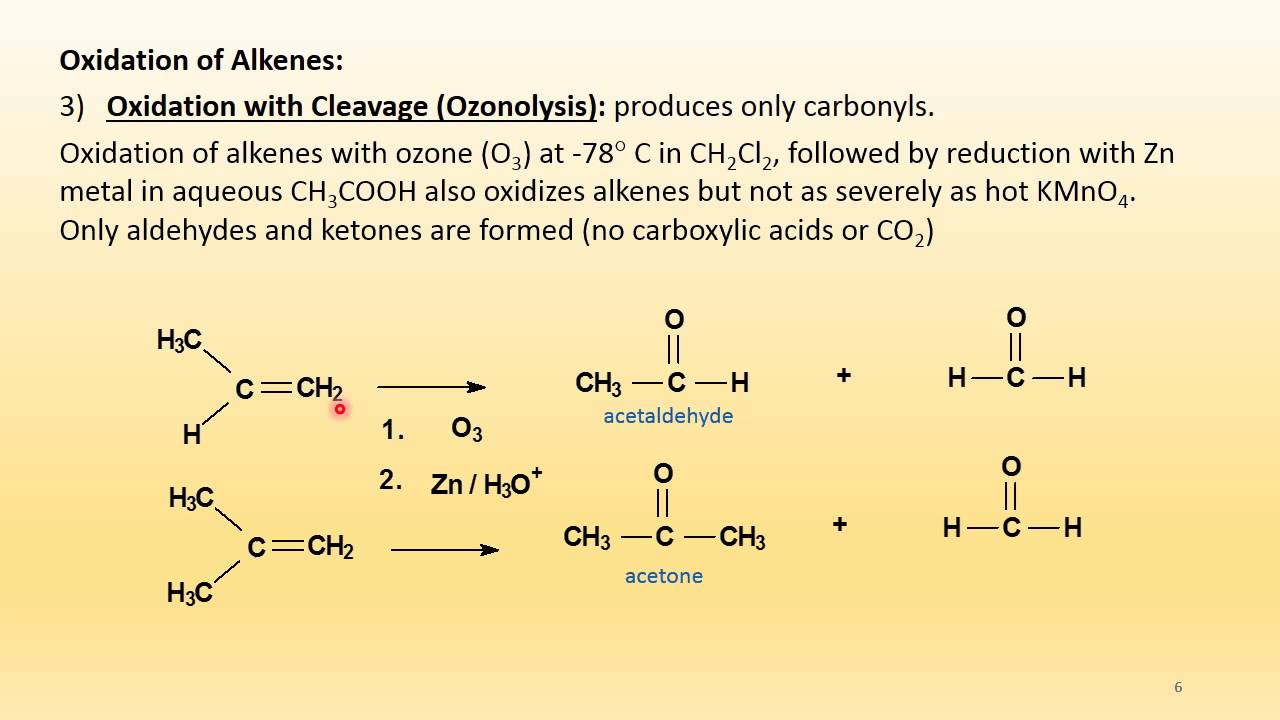
Alkenes can be cleaved by oxidation with ozone, o 3., using a process called ozonolysis. If you represent a simple alkene: Also know, is dehydrogenation oxidation or reduction? Is alkene to alkane reduction or oxidation?
One important alkene addition reaction is hydrogenation., where the alkene undergoes reduction to an alkane.
Alkanes can be prepared by reduction of haloalkanes. Alkenes are usually reduced by catalytic hydrogenation. Oxidation, followed by reduction, giving the more substituted alcohol. Acid and water (see above), but these simple addition conditions give rearrangements if a more stable carbocation is possible.
4.) protonation yields a neutral product.
This reaction is a reduction reaction. Groups that are cis on the alkene will end up cis on the cyclopropane product. Both hydrogen atoms are added to the same carbon of the double bond. Reduction and oxidation of alkenes:
3.) a carbanion is formed by the addition of an electron.
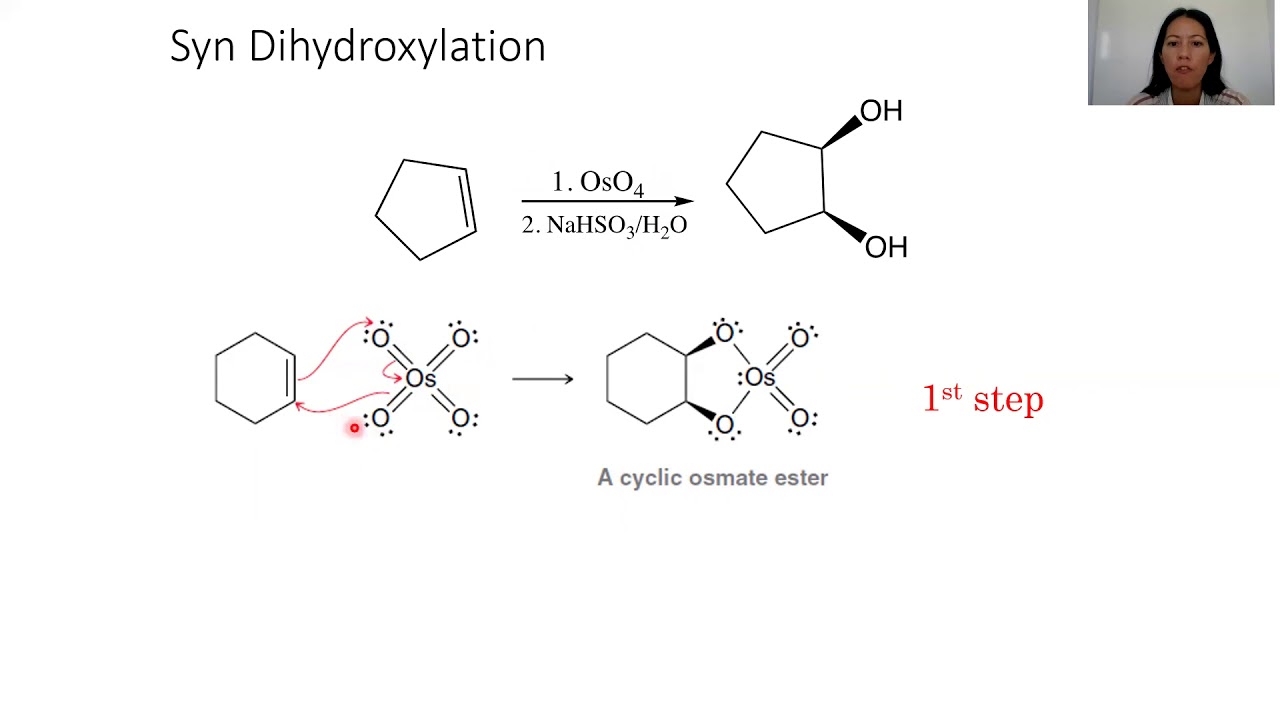
The alkane oxidation is an example of ligand accelerated catalysis, as hydroxide binding to oso4 is required for reaction. Buszek, now at the university of missouri, kansas city, has shown ( j. When an alkane is heated in the presence of an appropriate catalyst, it can be oxidized to the corresponding alkene in a reaction called a dehydrogenation reaction.