What are the four reactions of alkenes? The reaction takes place on the surface of the metal. The mechanism of halogenations occurs in three steps:
Organic Chemistry Reduction of Alkynes to Alkenes or
Further addition reactions of ethene.
This illustrates the principle of _____.
35) which of the following is the best reaction sequence to accomplish a markovnikov addition of water to an alkene with minimal skeletal rearrangement? Since water is not nearly acidic enough to protonate the double bond of an alkene by itself, you’ll need a strong acid as a catalyst. Elimination reactions that form alkenes. Chain initiation, chain propagation, and chain termination.
The mechanisms for the reactions are explained on separate pages.
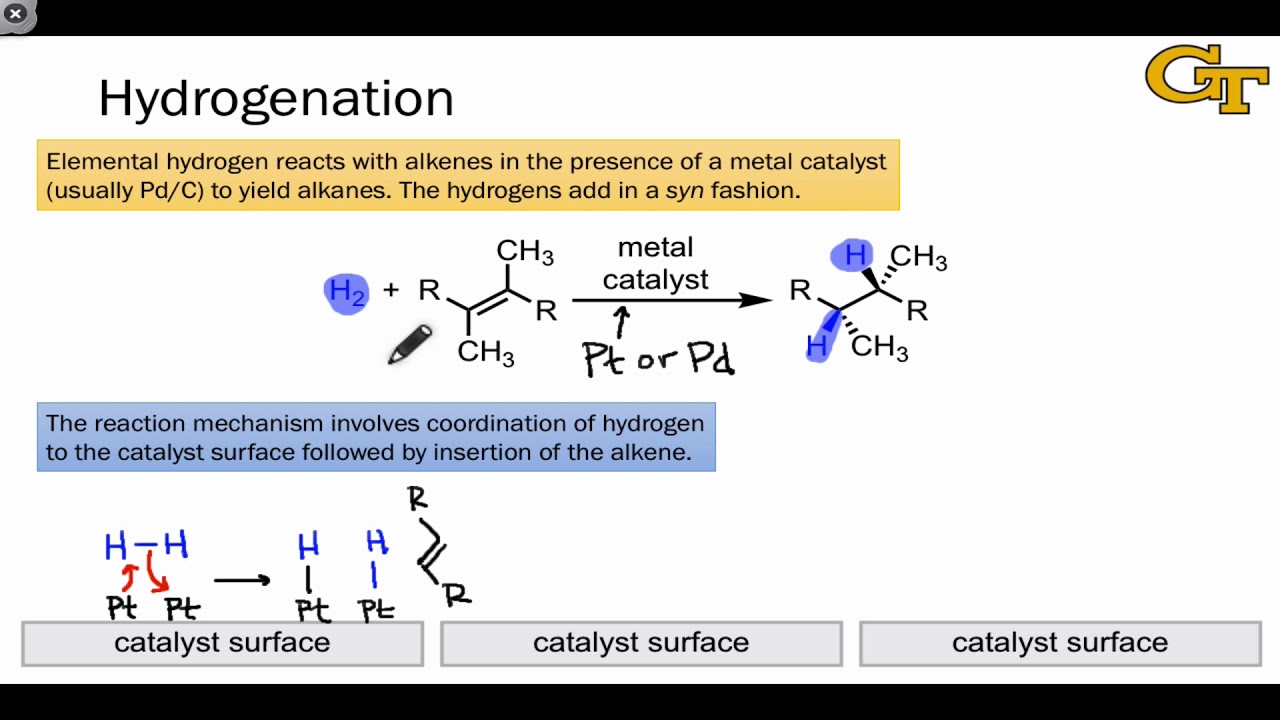
Reaction in which halogen is introduced into a molecule 3) hydration : This video takes you through the reaction and step by step mechanism for both a symmetrical starting alkene, and and asymmetrical starting alkene. Hydrogenation of an alkene yields an alkane. The mechanism for this reaction involves successive single electron transfers from the metal.
Ozone is bubbled through this solution at approximately 780 celsius.
Alkenes can undergo ozonolysis to form alcohols, aldehydes, ketones, or carboxylic acids. Complete step by step answer: This is followed by a step where ethanol, acting as a base, removes a proton from cβ of the carbocation. You would typically see something like sulfuric acid (h 2 so 4) as a catalyst in this reaction.
1) addition of hydrogen halides 2) halogenation :
What is the mechanism of halogenation of alkanes? An example of an alkene addition reaction is a process called hydrogenation.in a hydrogenation reaction, two hydrogen atoms are added across the double bond of an alkene, resulting in a saturated alkane.the heat released is called the heat of hydrogenation, which is an indicator of a molecule’s stability. The general procedure uses a solution of alkene in methanol. All alkenes undergo addition reactions with the hydrogen halides.
Addition reactions of alkenes are useful in organic synthesis.
Bond to complete conversion to alkene dehydration of alcohols step 1: Deprotonation occurs to form an alcohol. When the solution turns blue, the alkene is consumed (the blue colour comes from the unreacted ozone). Hydrogen molecules react with the metal atoms at the catalyst surface.
2.3 reactions of alkenes and alkynes ⇒ additions are the most common reactions using alkenes and alkynes addition to:
In a number of ways, these mechanisms are similar to the sn1 and sn2 mechanisms we described in chapter 7. For example, with ethene and hydrogen chloride, you get chloroethane: It doesn't decolourise bromine water.which is false mechanisms a level mechanism for thermal cracking (a level chemistry) electrophilic addition reactions We illustrate the e2 mechanism using the reaction of.
Water, being a nucleophile, attacks on the carbocation.
The alkyne to alkene reaction mechanism occurs in four steps, and this does not follow a catalytic mechanism: Reaction in which the elements of water (h and oh) are A hydrogen atom joins to one of the carbon atoms originally in the double bond, and a halogen atom to the other. Carbocation rearrangements may be observed.
The process of adding hydrogen across a double bond is sometimes referred to as hydrogenation.
In this reaction you end up adding water to your alkene. The 1 in e1 indicates that the rate determining step of the reaction The mechanism of hydrogenation this reaction is an example of a heterogeneous catalysis process. Alkene undergoes protonation to form carbocation by electrophilic attack of h3o+.
• the reaction uses h2 and a precious metal catalyst.
Alkenes are hydrocarbons used in products such as paint and plastics. Alkenes are reduced when treated with hydrogen gas in the presence of a nickel catalyst at 150°c. Explore the mechanism and explanation of the catalytic hydrogenation of alkenes. Lecture notes on the hydrogenation of alkenes :
This mechanism typically involves hydrobromination and hydrochlorination in an inert solvent.
Addition to symmetrical alkenes what happens?