121 rows molecular formula name of straight chain synonyms 1 1 1 ch 4: The name of the alkane is among the. Furthermore, it comprises a homologous series having a molecular formula of c nh2n + 2.
Question Video Applying the General Formula for Alkane
Total number of hydrogen atoms present in.
If we plug these values into the general chemical formula for alkanes, it shows that c10h18 follows the general chemical formula for.
8), butane (c 4 h. The most basic family of compounds has been called alkanes. C n h ( 2 n + 2 − 2 ∗ 0 − 4 ∗ 0 − 2 ∗ 0) c n h ( 2 n + 2 − 0 − 0 − 0) c n h ( 2 n + 2) ————————————————————————————————————————. This is a group of compounds consisting of carbon and hydrogen atoms with single covalent bonds.
The general formula for any specific group of hydrocarbons can be derived from this global formula.
Chain alkanes, cycloalkanes, and branched alkanes. The simplest alkane, methane, has one carbon atom and a molecular formula of ch 4. The boiling point of alkenes rises with an increase in molecular mass and a rise of 20 to 30 degrees is observed for each added carbon atom. An alkane with the molecular formula `c_(6)h_(14)` forms only three monochloro derivatives on chlorination.
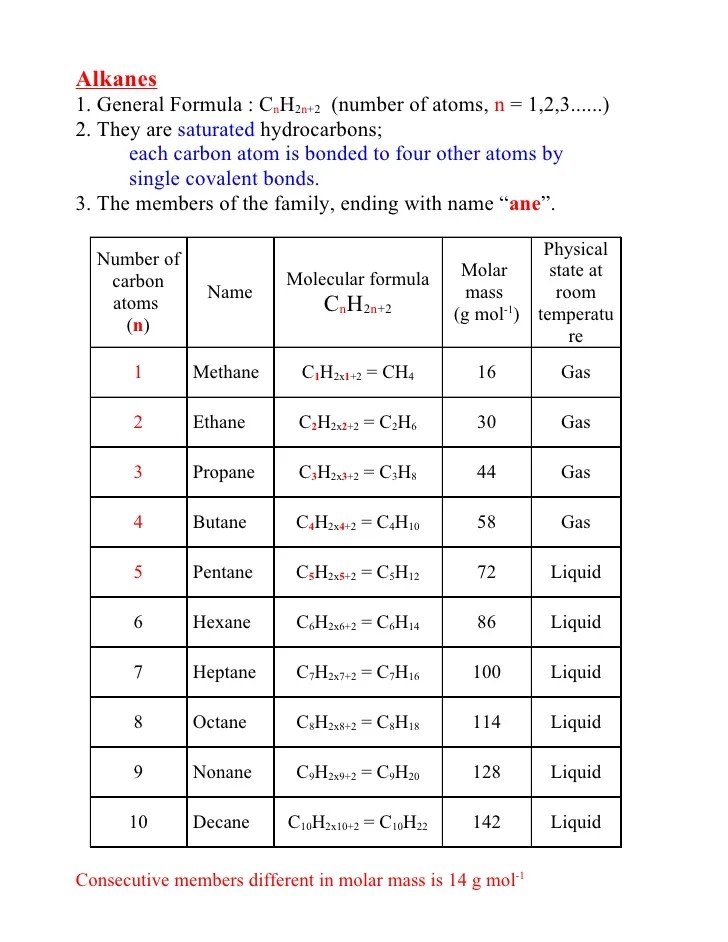
This group of compounds comprises a homologous series with a general molecular formula of c n h 2 n+2 , where equals any integer.
Propyl hydride 4 2 2 c 4 h 10: Skellysolve a 6 5 5 c 6 h 14: By saturated hydrocarbons, it means alkanes have single hydrogen and carbon atoms in their chemical formula. \[c_{n}h_{2n+2}\] the general formula means that the number of hydrogen atoms in an alkane is double the number of carbon atoms, plus two.
They comprise only hydrogen and carbon.
2n + 2 which can be deduced from the series. The general molecular formula of alkanes is {eq}c_nh_2n+2 {/eq}, where n is an integer number that represents the number of atoms in a compound. When we analyze several examples of alkanes, we can notice that they are made by a series of compounds containing carbon and hydrogen atoms with single covalent bonds. Alkenes are insoluble in water but quite soluble in organic solvents like benzene, ether, chloroform, etc.
Natural gas 2 1 1 c 2 h 6:
Alkenes are unsaturated hydrocarbons having a double bond between the carbon atoms. Methyl methane 3 1 1 c 3 h 8: They can be categorized into three groups which are: Find the molecular formula of the alkane represented in the mass spectrum.
Alkenes are often used as a synonym of olefin.
10), pentane (c 5 h. Adding the proper number of hydrogen atoms to each of the remaining carbon atoms, we have a molecule containing 10 carbon atoms each bonded by single bond, and 22 hydrogen atoms. As in alkanes, in alkenes also branching lowers the boiling point. The general formula of alkenes is c n h 2n.
Its molecular mass would be = 12 (n) + 1 (2n + 2) = 14n + 2 given molecular mass = 86 14n + 2 = 86?
Again by way of example, consider the series of alkanes: An alkane with the molecular formula c3h18 forms only one monochlorinated product when heated with cl2. Formula of alkane is c n h 2n+2. Molecular formula of the alkane is c6h14.
The formula for alkanes is cnh2n+2.
Methylethyl methane 5 3 3 c 5 h 12: The general formula of alkane is cnh2n+2. Propose a structure for the alkane. Their general formula is c n h 2n+2 for molecules which do not contain ring structures.
The general molecular formula of alkanes is {eq}c_nh_2n+2 {/eq}, where n is an integer number that represents the number of atoms in a compound.
The straight chain alkanes share the same general formula: The alkanes comprise a series of compounds that are composed of carbon and hydrogen atoms with single covalent bonds. The possible isomers for this molecular formula are: It can be seen how each compound differs from the following by 14 units of molecular weight, in addition to the general formula c.
For example in the diagram, the four hydrocarbon molecules contain 8 carbon atoms each.
Cnh replace the subscript letters with numbers. Condensed structural formula of the given alkane. The name olefin is derived from the greek word olefin gas, which means oil forming. In this case, n = 10 and g = 2.
Formula of alkane is c n h 2n+2.
6), propane (c 3 h. This formula can be used to write the correct. 100 43 90 c 70 50 85 57 30 20 29 10 71 100 10 20 30 40 50 0 80 90 100 110 m/z molecular formula: The chemical formula is c10h18.
Alkanes are in some respect the most boring of the organic compounds, since they are unreactive (mostly) towards acids, bases, oxidizing agents, reducing agents, and most of the other reagents that.
Alkanes are hydrocarbons in which the carbon atoms are held together by single bonds.



![Alkane Formula [with free study guide]](https://i2.wp.com/www.aceorganicchem.com/blog/wp-content/uploads/2017/08/Alkanes.png)