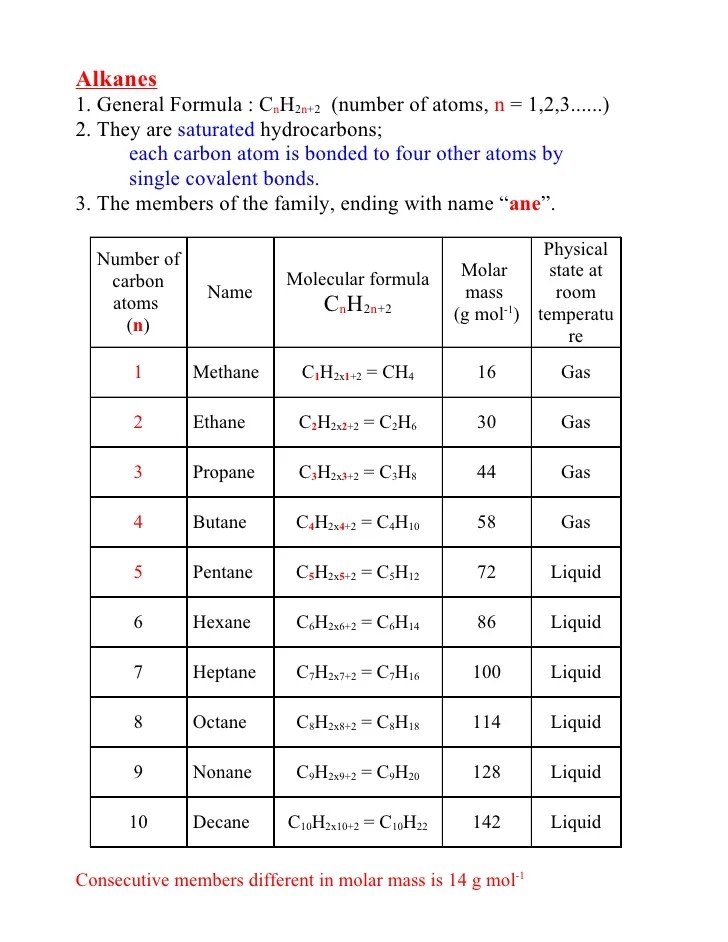
The general formula of alkanes is : The general formula for alkane is c n h 2 n + 2. N = number of carbon atoms in the same molecule.
PPT Hydrocarbons and Fuels PowerPoint Presentation ID
Only carbon, hydrogen and oxygen.
Thus it is known as unsaturated hydrocarbons.
(i) the number of alkane family represents the general formula cnh2n+2. This means that all the atoms share one pair of electrons. By reason of their formula alkanes are said to have no degrees of unsaturation. Alkanes have a general formula:
C x n h 2 n + 2.
The general formula for rings is c n h 2n. The general formula of alkanes is \({{\text{c}}_{\text{n}}}{{\text{h}}_{2{\text{n}} + 2}}\). C n h 2n+2 alkene: What is the general formula of alkynes?
Alkynes aliphatic hydrocarbons having general formula c.
Alkenes are hydrocarbons that contain carbon carbon double bond (c=c). If we take the general formula for an alkane, , then n is clearly 3. A fully saturated hydrocarbon, an alkane, has general formula cnh 2n+2: Alkenes have the general formula c n h 2n.
Alkanes have the general formula.
The alkanes are also called as paraffins. This formula can be used to write the correct. The general molecular formula of alkanes is {eq}c_nh_2n+2 {/eq}, where n is an integer number that represents the number of atoms in a compound. The general formula of alkenes are c n h 2n in comparison to alkanes with general formula c n h 2n+2.
Where the formula is cnh 2n or cnh 2nom, each 2 hydrogens less than 2n +2 represents a degree of unsaturation.
Alkenes are unsaturated hydrocarbons having a double bond between the carbon atoms. They are categorized into three types: This gives them a general formula : The general formula for the alkanes is c n h 2n+2 (where n is the number of carbon atoms in the molecule).
Write general formula of alkanes and four general methods for preparation of alkanes.
Alkanes are saturated with hydrogens, while alkenes are two hydrogen less than alkanes. Alkanes are saturated hydrocarbons, and they have a single covalent bond between hydrogen and carbon atoms. Some of alkanes are, c 3 h 8 propane c 5 h 1 0 pantane c 2 h 6 ethane c h 4 methane The name olefin is derived from the greek word olefin gas, which means oil forming.
Alkanes are acyclic aliphatic hydrocarbons having the general molecular formula c n h 2n + 2 [12].
Because they use only single bonds, alkanes contain the maximum number of hydrogen atoms relative to the number of carbon atoms, and are therefore said to be saturated. Alkenes are often used as a synonym of olefin. For cyclic alkanes, carbon must still have four bonds, hence each carbon is attached to two others and two hydrogens are groups may be attached to each carbon (structure on the right). The straight chain alkanes share the same general formula:
Hydrocarbons are molecules made from.
A brief structure shows only the bonds as in the compound on the extreme left. In an alkane, all 4 4 4 valencies of the carbon atom are satisfied with other hydrogen atoms. According to the formula, alkanes have hydrogen atoms. Chain alkanes, branched alkanes and cycloalkanes.
Methane gas is the first member of the homologous series of alkanes.
The most important is the first member of the series, the etino or acetylene of the formula hc≡ch. The general formula for an alkane is c n h 2n + 2. Alkanes have the general formula of c n h 2n + 2 where n is the number of carbon atoms. For example in the diagram, the four hydrocarbon molecules contain 8 carbon atoms each.
Substituting n for 3 gives the following:
C n h 2n alkyne: C x n h 2 n + 2.