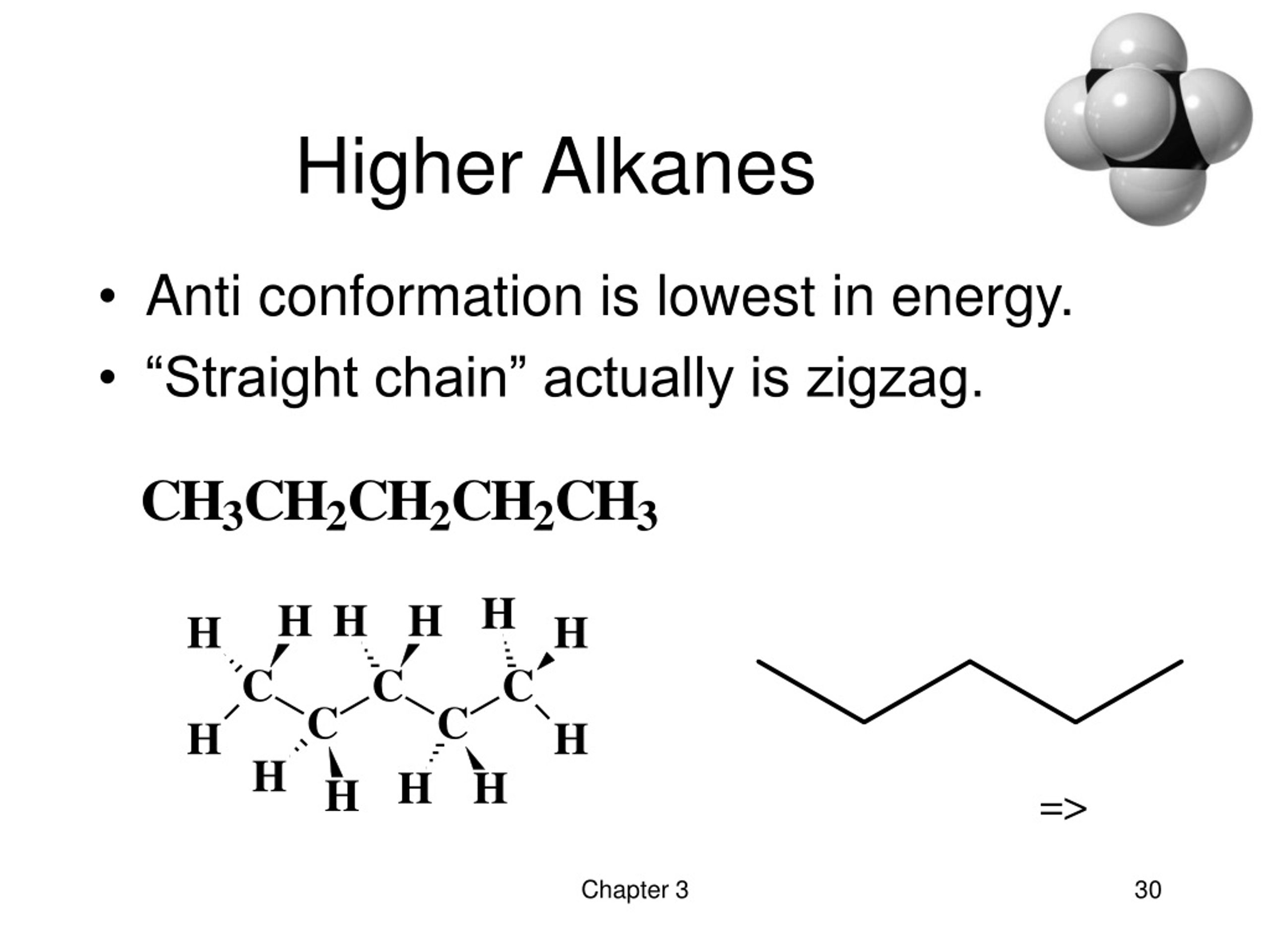
If molecule has functional groups, they determine the suffix alkyl group : Hydrogen higher than alcohol higher than alcohol polar acidic, volatile. Gas, next 12 are liquid, and the rest are solids.
Introduction to Organic Chemistry Alkanes Functional
An alkane is an organic compound that is composed only of carbon and hydrogen atoms.
Are known as functional groups.
Alkanes have the general formula cnh2n+2 c n h 2 n + 2 and can be subdivided into the following three groups: They can be viewed as molecular trees upon which can be hung the more active/reactive functional groups of biological molecules. This group of compounds consists of carbon and hydrogen atoms with single covalent bonds. Alkyl halides have a halogen atom as a functional group.
Official name of example (common name) formal name ending;
3.physical properties gradually change as you go down. The alkanes have two main commercial sources: An alkyl group is a functional group that. More specific propertiesand greater reactivitywith other compounds.
Nomenclature rules allow us to name alkyl halides and alcohols.
Functional groups also play an important part in organic compound nomenclature; Alkenes are an unsaturated form of hydrocarbons that are formed by double bonding between the carbon atoms. Petroleum (crude oil) and natural gas. These are known as saturated hydrocarbons.
Alkanes with lower molecular weight are gases.
Substitution and combustion reactions only alkene As molecular weight increases the alkanes stays as liquid or solid. The simplest alkene with one double bond is ethene (c 2 h 4 ). Physical properties of alkanones and alkanals are similar and are related to the polarity of the c=o functional group and the length of the carbon chain:
⚛ melting points and boiling points are greater than for alkanes of comparable alkane chain.
Also comprises a homologous series having a molecular formula of cnh2n+2. Alcohols have an oh group as a functional group. As a result, alkanes only dissolve in nonpolar solvents. Alkanes are the simplest family of.
Combining the names of the functional groups with the names of the parent alkanes provides a way to distinguish compounds.
They are generally abbreviated with the symbol for any. As the alkanes posses weak van der waals forces, the first four members, c 1 to c 4 are gases, c 5 to c 17 are liquids and those containing 18 carbon atoms or more are solids at 298 k. For branched cyclic alkanes, the position and name of the branch can be attached as suffix. When an alkane is mixed with a polar solvent, two distinct layers are formed where.
Hydrocarbon chain with one open point of attachment often r is used to describe a generic alkyl group.
They are colorless and odorless. Up to 24% cash back summary chart: Learn the properties and molecular formula of alkanes. All follow same general formula.
They are named using a prefix that.
2 neighboring members differ by ch2 group. The atoms of a functional group are linked together and to the rest of the compound by covalent bonds. Called functional groups, which are characteristic arrangement of atoms which define many of the physical and chemical properties of a class of organic compounds. Unsaturated, because they contain a c=c double bond, which means that they have two fewer hydrogen atoms than the corresponding alkane the c=c.
If all organic molecules were alkanes, then organic chemistry would.
Alkanes are a series of compounds that contain carbon and hydrogen atoms with single covalent bonds. In an elimination reaction, a double bond is formed as an hx or an hoh molecule is removed. Luckily, specific groups of atomsare found on organic molecules, which give the molecules. Chemical properties remain the same.