The lewis structure of methane can be generated by combining the four electrons in the valence shell of a neutral carbon atom with four hydrogen atoms to form a compound in which the carbon atom shares a total of eight valence electrons with the four hydrogen atoms. Skellysolve a 6 5 5 c 6 h 14: C n h 2n+2 each homologous series has its own general formula.
PPT Alkanes Structure and Conformation PowerPoint
−42 −188 2.01 (gas) 1 butane:
Alkanes which have long carbon chains are often called paraffins in chemical industry.
Chapter 1 alkanes 15 29 table 1.2 classes and functional groups of organic compounds class functional group example of expanded structural formula example of condensed iupac / common name alkane none h c h c h h h h ch3ch3 ethane alkene cc c ethene (ethylene) h h c h h h2cch2 alkyne cc c ch hc ch ethyne (acetylene) aromatic cc c c c c cc c c c c hh. Alkanes are the simplest hydrocarbons known to us. In organic chemistry, an alkane or paraffin is an acyclic saturated hydrocarbon. They can be categorized into three groups which are:
98 −91 684 (liquid) 9 octane:
121 rows molecular formula name of straight chain synonyms 1 1 1 ch 4: Table 22.1 on page 1059. Names start in “cyclo” (cyclopentane, cyclooctane, etc.) 7. In an alkane, all 4 4 4 valencies of the carbon atom are satisfied with other hydrogen atoms.
Melting point (°c) boiling point (°c) number of structural isomers:
36 −130 626 (liquid) 3 hexane: Apply this reaction chart for alkanes formulas in solving. \[c_{n}h_{2n+2}\] the general formula means that the number of hydrogen atoms. They comprise only hydrogen and carbon.
Reaction chart for alkanes formulas.
Methyl methane 3 1 1 c 3 h 8: The second alkane is ethane with the formula c 2 h 6. 69 −95 659 (liquid) 5 heptane: Ch 3 ch 2 ch 3.
−89 −183 1.26 (gas) 1 propane:
The general formula of alkanes is c n h 2n+2. Ch 3 (ch 2) 3 ch 3 Selected properties of the first 10 normal alkanes: Organic compounds having only two elements, carbon and hydrogen are called hydrocarbons.
Formula for cyclic alkanes c nh 2n
Methane gas is the first member of the homologous series of alkanes. This formula can be used to write the correct. The most simple alkane is methane with the formula ch 4. Boiling point [°c] melting point [°c] density [kg/m 3] (at 20 °c) isomers:
The general molecular formula of alkanes is {eq}c_nh_2n+2 {/eq}, where n is an integer number that represents the number of atoms in a compound.
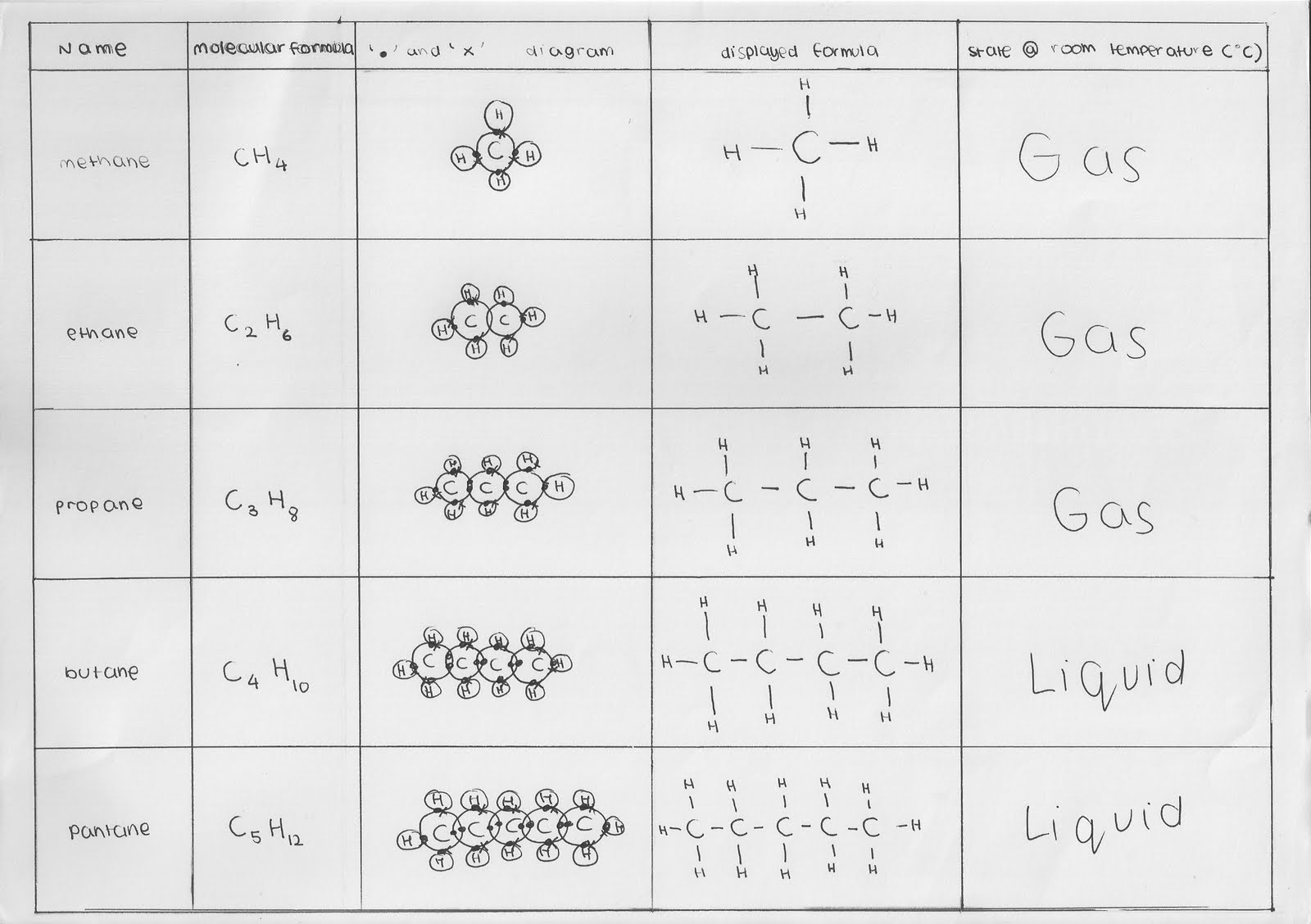
Alkanes are organic compounds consisting only of carbon and hydrogen (for this reason they belong to the broadest class of hydrocarbons), having the brute formula c n h. The first four alkanes are methane (ch 4), ethane (c 2 h 6), propane (c 3 h 8) and butane (c 4 h 10). They contain only sigma bond linkages between carbon and hydrogen. Ch4 alkanes cnh2n+2 carbon to carbon double bonds.
The straight chain alkanes share the same general formula:
Use 2 carbon atoms and 6 hydrogen atoms. The most basic family of compounds has been called alkanes. They are divided into two main classes, aliphatic and aromatic. For acyclic alkanes c nh 2n+2 • basically 2h per carbon (2n), plus 2 extra h’s at the ends (+2) • branched isomers for acyclic alkanes still have c nh 2n+2 6.
First member is methane (ch 4) also known as “marsh gas”, chief constituent of natural gas (~97%) hybridisation sp 3.
In an alkane is double the number of carbon atoms, plus two. The formula for alkanes is cnh2n+2. Propyl hydride 4 2 2 c 4 h 10: 0 −138 2.48 (gas) 2 pentane:
Natural gas 2 1 1 c 2 h 6:
Condensed structural formula of alkane. The alkanes are also called as paraffins. The table below shows the naming code for the stem. Table of contents what is the general formula of alkanes and cycloalkanes?
To use the general formula, replace n in the general formula with the number of c (carbon) atoms.
Structural formula chemical formula family general formula carbon to carbon single bonds. Use 2 carbon atoms and 4 hydrogen atoms. Ch 3 (ch 2) 2 ch 3. The molecular formula of alkane.
Next > ci ch24 0 ch o ch och.
Methylethyl methane 5 3 3 c 5 h 12: −162 −182 0.656 (gas) 1 ethane: C x n h 2 n + 2. The condensed structural formula of alkane.
In the alkanes chemical formula, they have only carbon and hydrogen atoms.
For example, butane may be written as ch 3 ch 2 ch 2 ch 3 or ch 3 (ch 2) 2 ch 3. Chain alkanes, cycloalkanes, and branched alkanes. Ethane is an alkane, the stem “eth” tell us that it has 2 carbon in the molecule, and the suffix “ane” indicates that it is a member of the alkanes homologous series. The general formula for an alkane is c n h 2n+2 where n is the number of carbon atoms in the molecule.
They have a general formula of c n h 2n+2.
There are two ways of writing a condensed structural formula. C x n h 2 n + 2. The simplest alkane is methane:





.PNG)