What are the names of the alkanes of the homologous linear chain series up to 10 carbon atoms? Alkanes that have 5 or more carbons are named using prefixes that indicate the number of carbons. For example, an alkane with eight carbon atoms has the molecular formula c.
Alkane Nomenclature YouTube
Any molecule with this structure is going to have the formula c n h n+2 , where n is any integer.
Organic compounds that come from crude oil that have at least 1 carbon carbon double bond (c=c) homologous series:
Again by way of example, consider the series of alkanes: Ch 3 ch 2 ch 2 ch 2 ch 2 ch 3. They contain only sigma bond linkages between carbon and hydrogen. Molecular formula (c n h 2n + 2) condensed structural formula.
It can be seen how each compound differs from the following by 14 units of molecular.
An alkane is a simple hydrocarbon containing carbon and hydrogen single bonded to each other, with a carbon backbone. Condensed structural formula of alkane. Methane, propane, ethane, and butane are four alkanes. Formula of alkane is c n h 2n+2.
Alkanes belong to the family of saturated hydrocarbons that is;
For acyclic alkanes c nh 2n+2 • basically 2h per carbon (2n), plus 2 extra h’s at the ends (+2) • branched isomers for acyclic alkanes still have c nh 2n+2 6. They can be categorized into three groups which are: Number of carbons molecular formula structural formula name of alkane 1 ch 4 ch 4 methane 2 c 2 h 6 ch 3 ch 3 ethane 3 c 3 h 8 ch 3 ch 2 ch 3 propane 4 c 4 h 10 ch 3 (ch 2) 2 ch 3 n. Iupac nomenclature of alkanes, alkynes, and alkenes are explained below:
They comprise only hydrogen and carbon.
The first four names come from the names methanol, ether, propionic acid, and butyric acid. Every hydrogen is joined to a carbon. The carbon in methane is sp^3 hybridized. Using this formula, we can write a molecular formula for any alkane with a given number of carbon atoms.
10 ), pentane (c 5 h.
C n h 2n+2 each homologous series has its own general formula. Formula ( cnh2n+2 ) 1. The first four alkanes are methane (ch 4), ethane (c 2 h 6), propane (c 3 h 8) and butane (c 4 h 10). Names start in “cyclo” (cyclopentane, cyclooctane, etc.) 7.
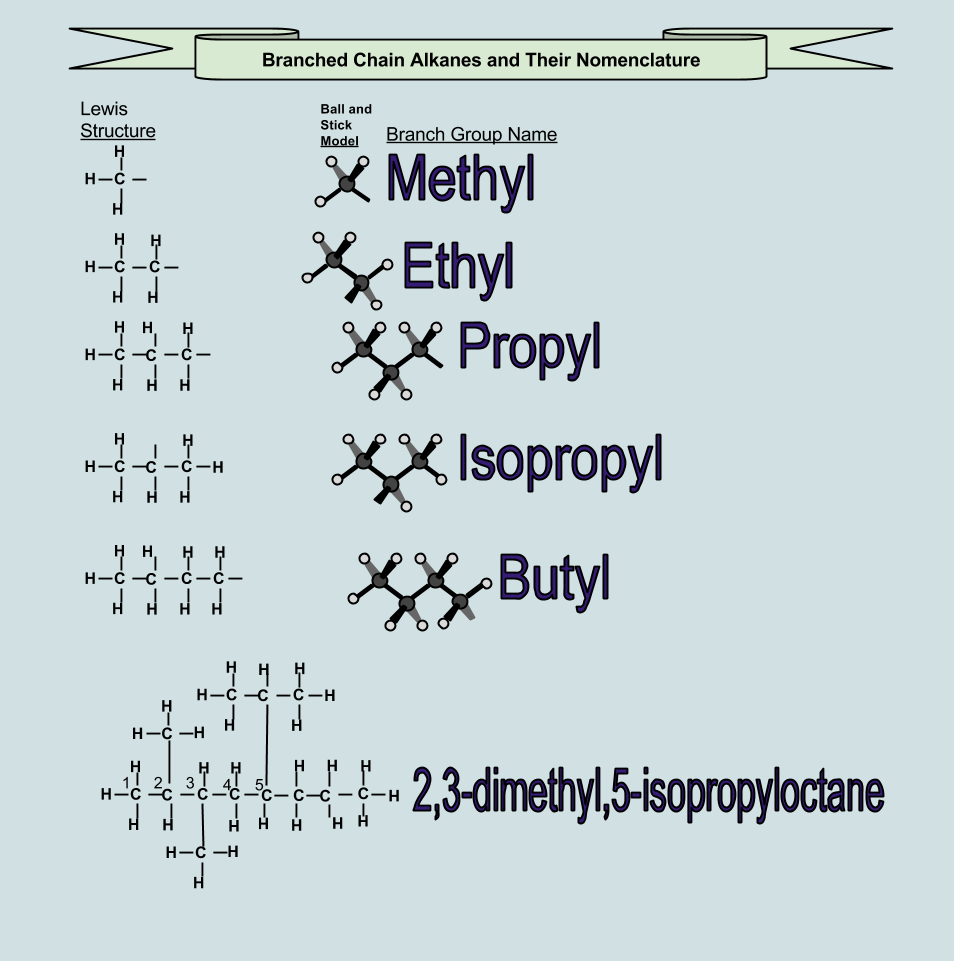
Root names are used with various endings to indicate branches, type of bonds between carbons, and functional groups.
Chain alkanes, cycloalkanes, and branched alkanes. 6 ), propane (c 3 h. C n h 2 n + 2. Methane (ch 4 ), ethane (c 2 h.
To use the general formula, replace n in the general formula with the number of c (carbon) atoms.
Same formula, but the atoms are bonded together in a different order 2. Number of carbons (n) name. The simplest alkane is the gas methane, whose molecular formula is ch 4. The following list gives the most basic root the with normal hydrocarbon alkane endings for the number of carbons in the longest continuous chain.
Methane, propane, ethane, and butane are four alkanes.
Alkanes have the general chemical formula c n h 2n+2. Formula for cyclic alkanes c nh 2n 8 ), butane (c 4 h. They have a general formula of c n h 2n+2.
Structures and names of alkanes.
113 rows formula common name synonyms 1 ch 4: The condensed structural formulas of the first ten straight chain alkanes and their iupac names are listed below: The principle of homology allows us to write a general formula for alkanes: A series of compounds with the same functional group and similar chemical properties.
The formula for alkanes is cnh2n+2.
Molecular formula name of straight chain synonyms 1 1 1 ch 4: Which is the formula for an alkane hydrocarbon? The most basic family of compounds has been called alkanes. Alkanes are the simplest hydrocarbons known to us.
The first 10 alkanes # of carbons name formula (cnh2n+2) 1 methane ch 4 2 ethane c 2h 6 3 propane c 3h 8 4 butane c 4h 10 5 pentane c 5h 12 6 hexane c 6h 14 7 heptane c 7h 16 8 octane c 8h 18 9 nonane c 9h 20 10 decane c 10h 22 b.
Also to know is, what are the 20 alkanes? Compounds that have a carbon carbon (c=c) double bond (like alkenes) single covalent bond:




![Alkane Formula [with free study guide]](https://i2.wp.com/www.aceorganicchem.com/blog/wp-content/uploads/2017/08/Alkanes.png)