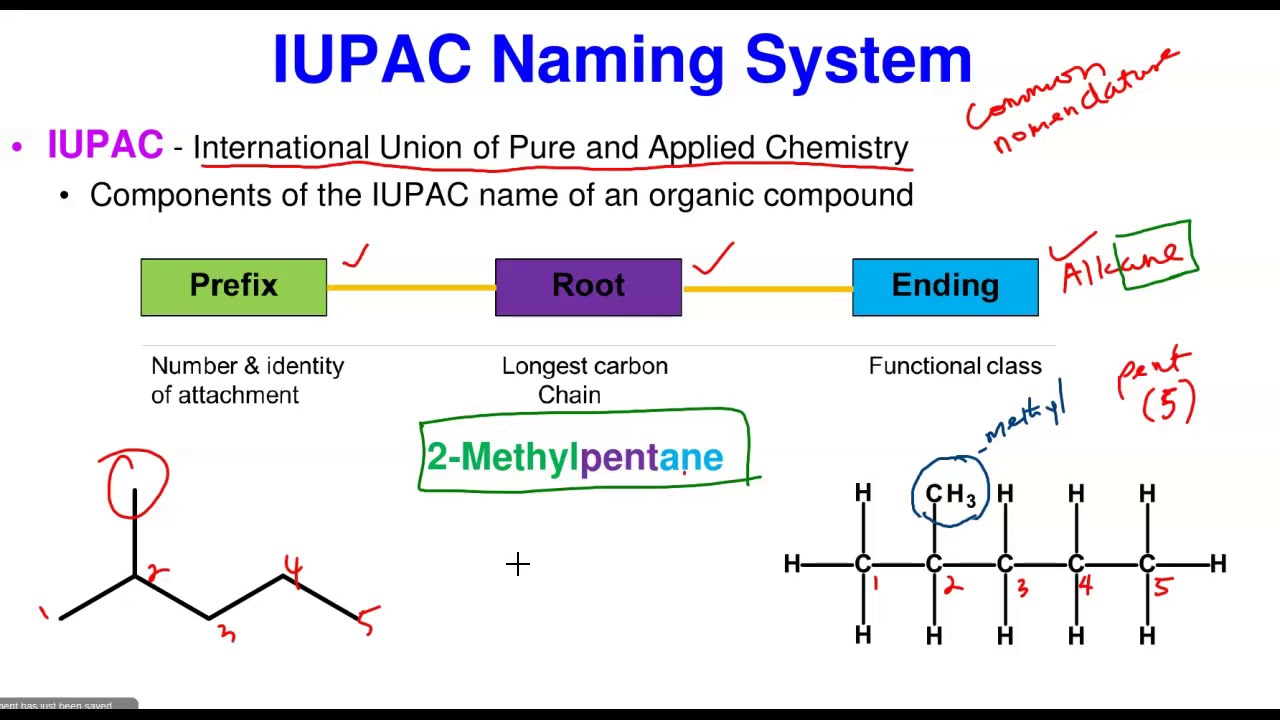
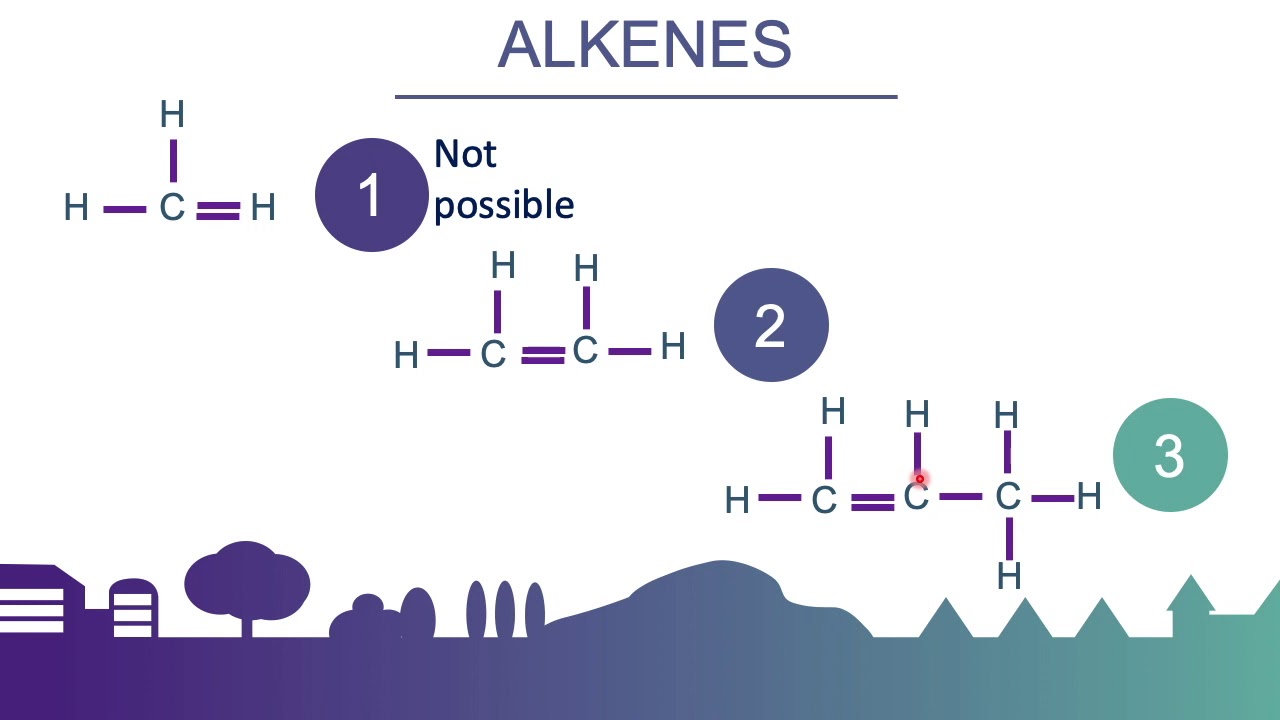
Alkenes and alkynes can be transformed into almost any other functional group you can name! Alkanes, alkenes, and alkynes are all organic hydrocarbons. In alkenes, the carbon atom where the double bond is present represents the number with the alkene.
Synthesis (5) Reactions of Alkynes Master Organic
And alkynes have triple bonds between carbons.
Alkanes, alkenes and alkynes mcqs alkanes have single bonds between carbons in a hydrocarbon.
• of the series of compounds alkane, alkene, and alkyne, the carbon of a terminal alkyne has the most s character (50%). It is an alkane so it is saturated. Identify each as an alkane, an alkene, or an alkyne. Many of these molecules are used in the production of other materials, such as plastics, but their main use is as a fuel source.
Also referred to as paraffin.
There are only single bonds between the hydrogen and carbon. Alkenes have double bonds between carbons. Alkynes are unsaturated carbon that shares a triple bond at the carbon site. Alkenes are generally colorless apolar compounds, somewhat similar to alkanes but more reactive.
Difference between alkanes, alkenes and alkynes alkanes, alkenes vs alkynes alkanes, alkenes and alkynes are all hydrocarbons with different structures and thus different physical and chemical properties.
The simplest alkene, ethylene (c 2 h 4) (or ethene in the iupac nomenclature) is the organic compound produced on the largest scale industrially. Unsaturated hydrocarbons have double or triple bonds and are quite reactive; Such electrophilic carbons can undergo nucleophilic substitution or elimination reactions, or both, depending upon the structures of the reacting molecules, the strength of the nucleophile. Alkynes are unsaturated carbon that shares a double bond at the carbon site.
Reaction in which the elements of water (h and oh) are
Before understanding each of these 3 types, you need to know that alkanes, alkenes & alkynes are hydrocarbons. This catalysts which are finely divided is like. Briefly identify the important distinctions between an alkene and an alkane. > the first step is usually the free radical halogenation of an alkane.
In this process, dihydrogen gas is added to alkynes and alkenes in the present catalyst.
Alkanes are solid, liquid or gas at room temperature depending on the size of their molecules.to learn detailed structures, formulas, and physical properties of alkanes with faqs and videos, visit byju’s for more information. Of alkyl groups attached to the double bonded carbon atom. Organic chemistry alkene and alkyne addition reactions introduction to reactions and mechanisms. C 2 h 4 + 3o 2 → 2co 2 + 2h 2 o.
A quick way to recognize an alkane is the general formula:
This means that alkynes can be reduced by the addition of one or two equivalents of h2, to alkenes and. Alkanes are simplest organic compounds that consist of single bonded carbon and hydrogen atoms with the general formula cnh2n+2. 1) addition of hydrogen halides 2) halogenation : We will review their nomenclature, and also learn about the vast possibility of reactions using alkenes and alkynes as starting materials.
Alkynes as explained, since there is a bigger volume to an alkane than its corresponding alkyne (i.e.
Since alkenes are hydrocarbons, they burn in oxygen to give water and carbon dioxide. An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon is a molecule which only contain the atoms hydrogen and carbon. The first few members of the series are gases or liquids at room temperature. The alkanes are also called as paraffins.
This gives them a general formula :
There are only single bonds between the carbon. C x n h 2 n + 2. For example, all the above unbranched alkenes shown had double bond at the first atom. In an alkane, all 4 4 4 valencies of the carbon atom are satisfied with other hydrogen atoms.
The single bond is made.
Reaction in which halogen is introduced into a molecule 3) hydration : In case of dehydrohalogenation, that alkene is the preferred product which has more no. Alkenes and alkynes can also be halogenated with the halogen adding across the double or triple bond, in a similar fashion to hydrogenation. From dihalogen derivative (vicinal dihalides) from alkynes:
Therefore, a terminal alkyne anion is the most stable of the series, and a terminal alkyne is the most acidic.
The halogenation of an alkene results in a dihalogenated alkane product, while the halogenation of an alkyne can produce a tetrahalogenated alkane. When a carbon is bonded to one or more electronegative atoms, it takes on a partial positive charge and it is electrophilic. A quick way to recognize an alkene is its general formula: Methane gas is the first member of the homologous series of alkanes.
The general formula of the homologous series of alkanes is cnh2n + 2 where n is an integer.
C x n h 2 n + 2. Alkyne reduction by lindlar’s catalyst or na/nh3. Hydrocarbons in the study of organic chemistry, the organic compounds which are made up of carbon and hydrogen are called hydrocarbons. Alkanes are a group of acyclic, saturated hydrocarbons.
Alkane can be prepared from alkene and alkyne through the process of hydrogenation.
Each alkene has 2 fewer electrons than the alkane with the same number of carbons. Hydrocarbons are organic compounds which only consist of carbon (c) and hydrogen (h) as their elements. Alkene alkyne four major additions: Alkanes can be obtained by hydrogenation of alkenes by means of hydrogen and catalyst.
With the same number of carbons) the alkane should have a.