C x n h 2 n + 2. Also referred to as paraffin. Ch 3 (ch 2) 28 ch 3.
CH105 Chapter 7 Alkanes and Halogenated Hydrocarbons
Reaction in which halogen is introduced into a molecule 3) hydration :
For acyclic alkanes c nh 2n+2 • basically 2h per carbon (2n), plus 2.
For alkanes with three or less than three carbon atoms. This gives them a general formula : Therefore, alkanes are refered to as a homologous series. Acetylene hc ≡ ch −84.
Propyne hc ≡ cch3 −23.
Since there are no double or triple bonds, alkanes are called saturated hydrocarbons. Autoignition temperature = kindling point Boiling points of alkenes and alkynes. The first few members of the series are gases or liquids at room temperature.
From 5 up, come from greek 4.
1) addition of hydrogen halides 2) halogenation : Alkanes are also known as paraffin. In simple alkanes, which have very few bands, each band in the spectrum can be assigned. Boiling points of alkenes and alkynes.
• of the series of compounds alkane, alkene, and alkyne, the carbon of a terminal alkyne has the most s character (50%).
C x n h 2 n + 2. Identify each as an alkane, an alkene, or an alkyne. Each alkane is different from previous by one carbon and two hydrogens. Ch 3 (ch 2) 18 ch 3.
More c’s high boiling point (london force) 5.
From above table, you may see melting and boiling points of. All these atoms are linked to each other via single covalent bonds. The below lists the first 20 alkanes, although there are many more. Alkene alkyne four major additions:
The longest continuous chain of an alkane is called the parent chain.
In an alkane, all 4 4 4 valencies of the carbon atom are satisfied with other hydrogen atoms. Boiling points of alkenes and alkynes. The alkanes are also called as paraffins. Chapter 1 alkanes 15 29 table 1.2 classes and functional groups of organic compounds class functional group example of expanded structural formula example of condensed iupac / common name alkane none h c h c h h h h ch3ch3 ethane alkene cc c ethene (ethylene) h h c h h h2cch2 alkyne cc c ch hc ch ethyne (acetylene) aromatic cc c c c c cc c c c.
Classify each compound as saturated or unsaturated.
In case of dehydrohalogenation, that alkene is the preferred product which has more no. Methane gas is the first member of the homologous series of alkanes. 2.3 reactions of alkenes and alkynes ⇒ additions are the most common reactions using alkenes and alkynes addition to: From dihalogen derivative (vicinal dihalides) from alkynes:
The simplest alkene, ethylene (c 2 h 4) (or ethene in the iupac nomenclature) is the organic compound produced on the largest scale industrially.
Of alkyl groups attached to the double bonded carbon atom. Alkynes are unsaturated carbon that shares a double bond at the carbon site. Alkynes are unsaturated carbon that shares a triple bond at the carbon site. Alkanes are the simplest hydrocarbon chains.
Briefly identify the important distinctions between an alkene and an alkane.
Each type of fuel will have mixtures of different alkane chains, and these mixtures will give the fuel its desired properties. Alkanes are called hydrocarbons because they are composed of c and h atoms. The general formula for alkanes is c n h 2n+2. The ir spectrum of octane is shown below.
Name formula boiling point (° c ) ethylene ch2 d;
Unsaturated hydrocarbons have double or triple bonds and are quite reactive; Alkanes are saturated as they are linked only by single bonds. Here is a list of the first 10 alkanes. Reaction in which the elements of water (h and oh) are
Therefore, a terminal alkyne anion is the most stable of the series, and a terminal alkyne is the most acidic.
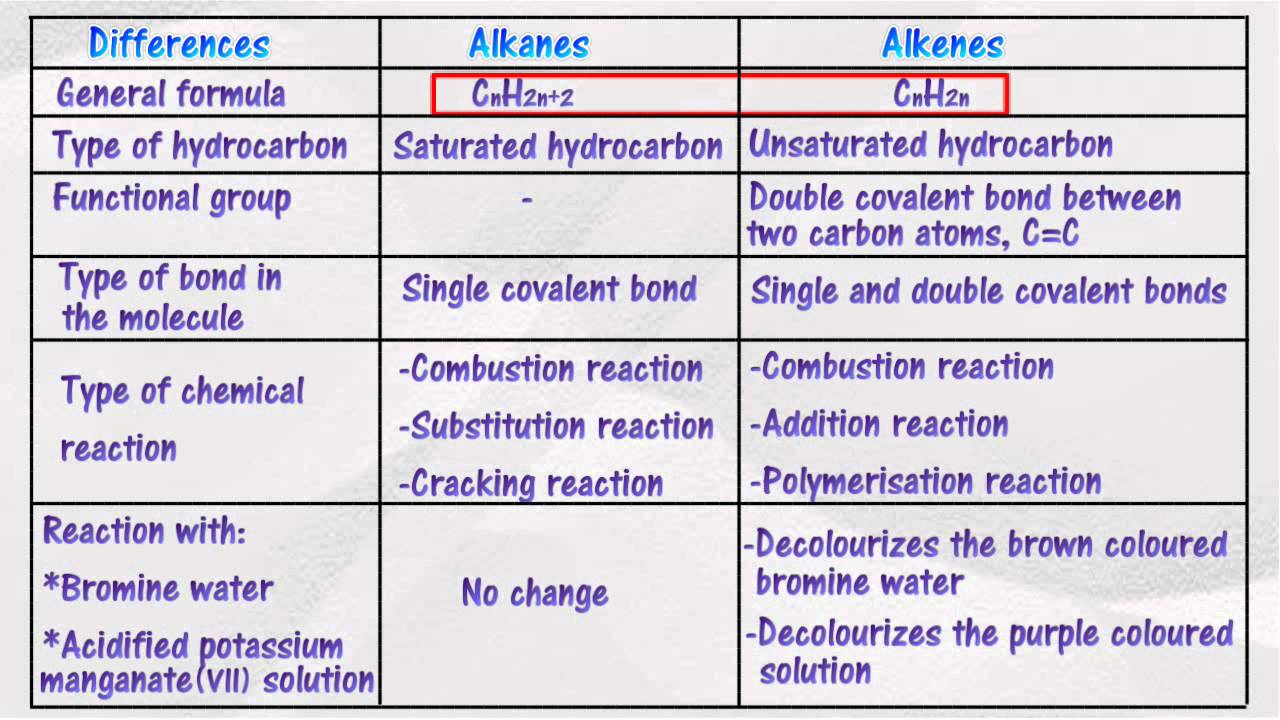
Alkanes are saturated hydrocarbons having the chemical formula c n h 2n+2 (where n is a whole number). Alkenes are generally colorless apolar compounds, somewhat similar to alkanes but more reactive. Alkanes are simplest organic compounds that consist of single bonded carbon and hydrogen atoms with the general formula cnh2n+2. Alkanes vs alkenes vs alkynes.
Names all end in “ane” 3.
These are commonly known as paraffins and waxes. Mccord's online textbook resource for ch301n and ch302n (including some alkane properties, section 3.5) 1. Chain alkanes, cycloalkanes, and branched alkanes are the three subgroups of alkanes.

![Alkane Formula [with free study guide]](https://i2.wp.com/www.aceorganicchem.com/blog/wp-content/uploads/2017/08/Alkanes.png)



