Reaction in which the elements of water (h and oh) are 2.3 reactions of alkenes and alkynes ⇒ additions are the most common reactions using alkenes and alkynes addition to: For acyclic alkanes c nh 2n+2 • basically 2h per carbon (2n), plus 2 extra h’s at the ends (+2) • branched isomers for acyclic alkanes still have c nh 2n+2 6.
PPT 20.7 Naming Alkenes & Alkynes PowerPoint
Many alkynes have been found in nature.
Discusses how to write the structural formula of the hydrocarbons alkanes, alkenes and alkynes.
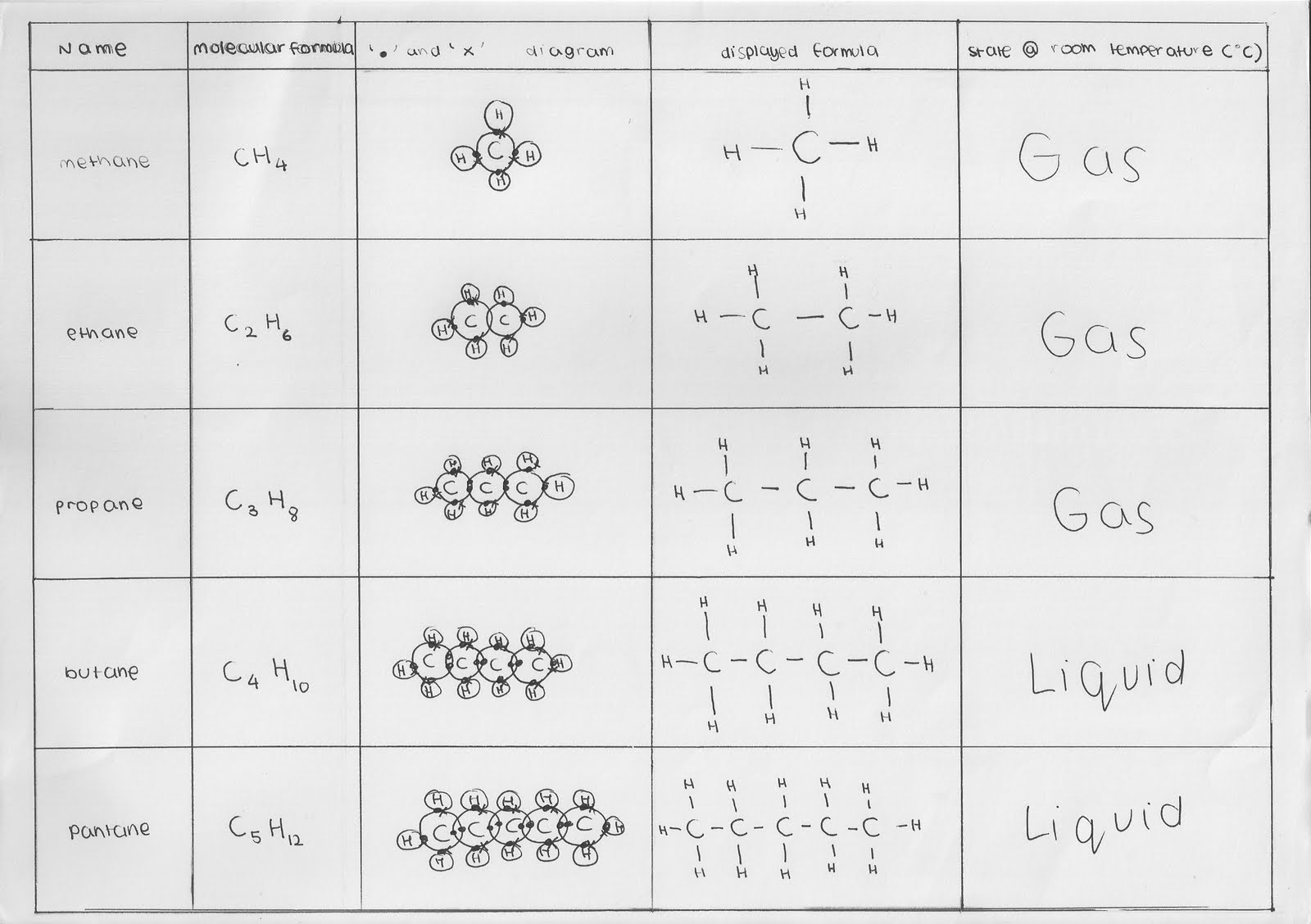
Ethyne (c 2 h 2) is the first member of the alkyne family, with two carbon atoms connected by a triple bond. Methane gas is the first member of the homologous series of alkanes. Where n is number of carbon atoms. Here n is the number of atoms of carbon in their chemical structure.
The alkanes are also called as paraffins.
The alkynes are unsaturated hydrocarbons that contain one triple bond, the general formula of alkynes c n h 2n+2 and the triple bond is known as the ‘acetylenic bond’. General formula of alkene is c n h 2n; This group of compounds comprises a homologous series with a general molecular formula of c n h 2 n+2 , where equals any integer. C x n h 2 n + 2.
Reaction in which halogen is introduced into a molecule 3) hydration :
Formula for cyclic alkanes c nh 2n C x n h 2 n + 2. The simplest alkane, methane, has one carbon atom and a molecular formula of ch 4. (c5h12), we observe that it is possible to write other structural formulas in addition to those of the two compounds mentioned:
Alkanes can be obtained by hydrogenation of alkenes by means of hydrogen and catalyst.
The typical isomeries of alkanes are the structure (or chain) isomerism and the conformational isomerism. C n h 2n each homologous series has its own general formula. The alkanes comprise a series of compounds that are composed of carbon and hydrogen atoms with single covalent bonds. The general formula is cnh2n, the same as cycloalkanes.
For example, would the condensed structural formula of butene be ch2=chch2ch3 or ch2chch2ch3?
A fully saturated hydrocarbon, an alkane, has general formula cnh 2n+2: By reason of their formula alkanes are said to have no degrees of unsaturation. So accordingly the number of hydrogen atoms is 2n+2. Alkenes undergo self addition in which alkene molecules join together to form long chains called polymers.
The simplest alkane is methane which is ch 4.
If c = 1 then c n h 2n = c 1 h 2x1 = ch 2 name of this compound: This chemical formula will stand true for all saturated hydrocarbons. I was wondering for condensed structural formulas of alkenes and alkynes, do the double and triple bonds have to be shown? To use the general formula, replace n in the general formula with the number of c (carbon) atoms.
Up to 24% cash back alkenes •alkenes have a double bond.
In an alkane, all 4 4 4 valencies of the carbon atom are satisfied with other hydrogen atoms. The general formula of the homologous series of alkanes is cnh2n + 2 where n is an integer. The formula for alkanes is cnh2n+2. The general formula for alkanes is c n h 2n+2.
Learning goal write the iupac names for alkanes with substituents and draw their condensed structural formulas and skeletal formulas.!
C n h 2n+2 each homologous series has its own general formula. Also, just to confirm, the condensed structural formula and structural formula would be the same for butene, correct? Alkene alkyne four major additions: Example of an alkene eg:
11.3 alkanes with substituents when an alkane has four or more carbon atoms, the atoms can be arranged so that a side group called a branch or substituent is attached to a carbon chain.
Names start in “cyclo” (cyclopentane, cyclooctane, etc.) 7. C 11h 24 line bond structural formula: Since, hydrocarbon having one carbon atom is known as methane. They can be categorized into three groups which are:
To use the general formula, replace n in the general formula with the number of c (carbon) atoms.
A) alkenes that contain 4 carbon atoms (three possible) b) cyclic alkenes that contain 4 carbon atoms (three possible) c) alkynes that contain 4 carbon atoms (two possible, neither of them are cyclic alkynes) 2. 1) addition of hydrogen halides 2) halogenation : This gives them a general formula : Here one atom of carbon is bonded to four atoms of hydrogen with single bonds.
10 best images of simple organic compounds
What is the general formula of alkanes and cycloalkanes?