Alkanes are saturated hydrocarbon while alkanes and alkynes are unsaturated hydrocarbons. Alkene alkyne four major additions: In the alkanes chemical formula, they have only carbon and hydrogen atoms.
Naming Alkanes Nicole Ling Brilliant
2.3 reactions of alkenes and alkynes ⇒ additions are the most common reactions using alkenes and alkynes addition to:
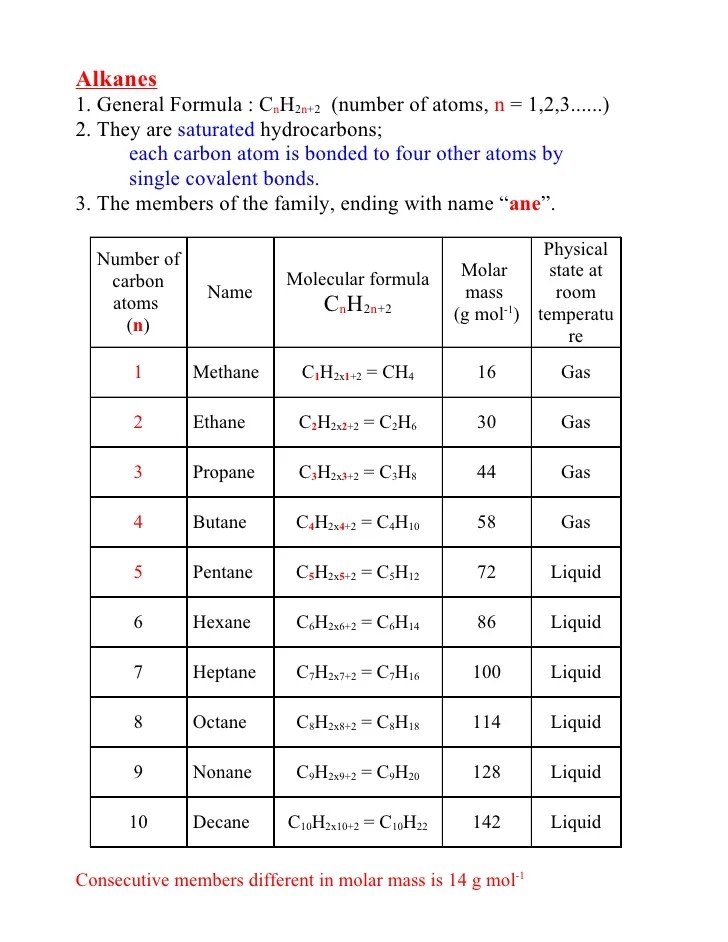
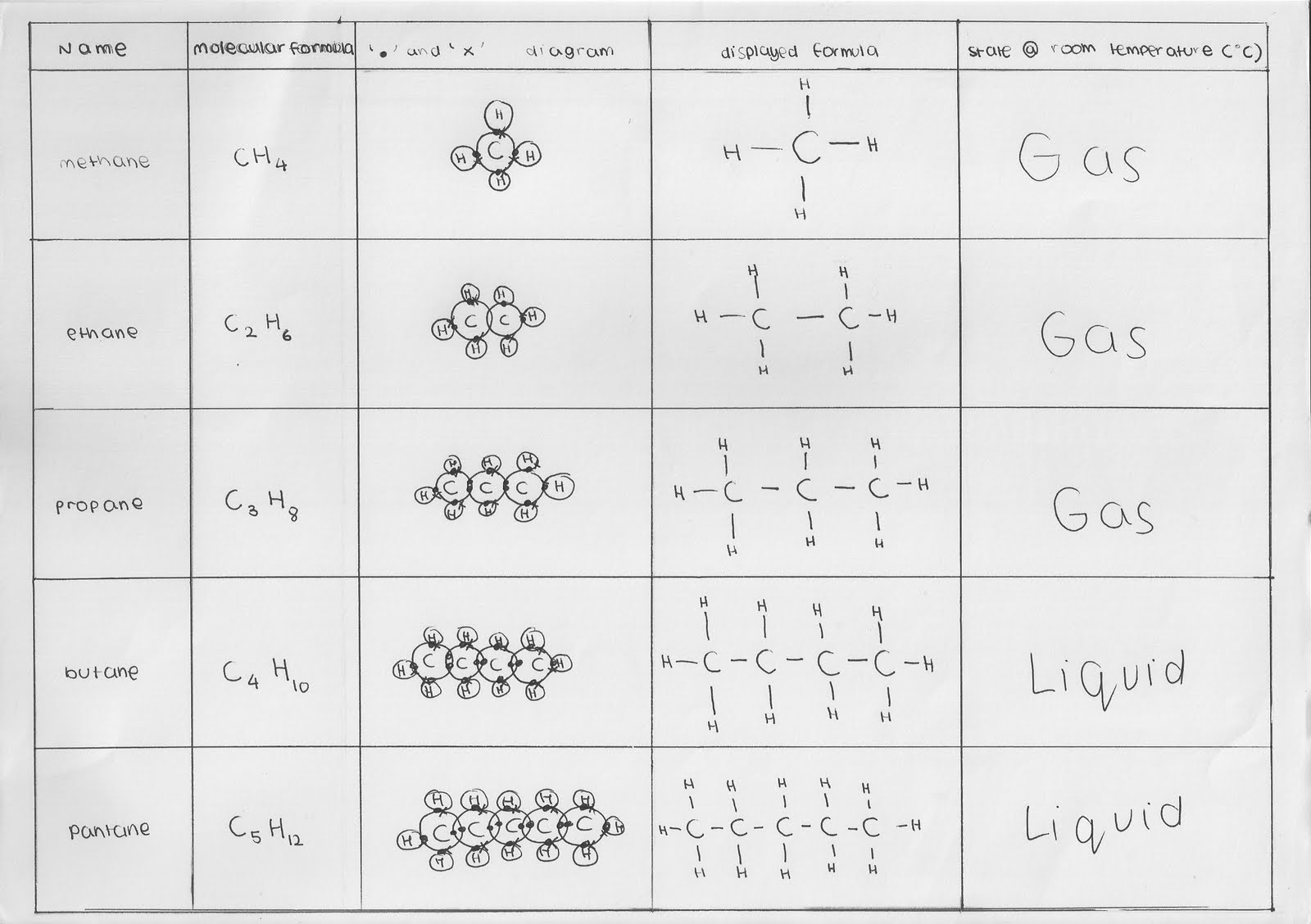
Methane gas is the first member of the homologous series of alkanes.
In an alkane, all 4 4 4 valencies of the carbon atom are satisfied with other hydrogen atoms. Formula state (25°c) bp (°c) methane: For alkanes with three or less than three carbon atoms. Reaction in which the elements of water (h and oh) are
Alkanes form bonds with carbon or hydrogen atoms.
Alkanes have the general formula c n h 2n+2 and can be subdivided into the following three groups: The presence of double bonds in alkenes makes them highly reactive chemically. Chapter 1 alkanes 15 29 table 1.2 classes and functional groups of organic compounds class functional group example of expanded structural formula example of condensed iupac / common name alkane none h c h c h h h h ch3ch3 ethane alkene cc c ethene (ethylene) h h c h h h2cch2 alkyne cc c ch hc ch ethyne (acetylene) aromatic cc c c c c cc c c c c hh. In an alkane is double the number of carbon atoms, plus two.
(c5h12), we observe that it is possible to write other structural formulas in addition to those of the two compounds mentioned:
The boiling point increases with the increase in the molecular weight. For acyclic alkanes c nh 2n+2 • basically 2h per carbon (2n), plus 2 extra h’s at the ends (+2) • branched isomers for acyclic alkanes still have c nh 2n+2 6. Olefins • steroids • hormones • biochemical regulators For example, an alkane with 2(n) carbon atoms, will have 6 (2n+2) hydrogen atoms.
Introduction alkenes hydrocarbons containing c=c old name:
The general formula for alkanes is c n h 2n+2. An alkene has a double bond; 1) addition of hydrogen halides 2) halogenation : Apply this reaction chart for alkanes formulas in solving the alkane chemical reactions and.
Straight chained alkanes have higher boiling points.
Melting point of alkanes also increases with the increase in molecular weight. Alkenes are often used as a synonym of olefin. Contains a carbon‐carbon triple bond and has the general formula cnh2n‐2. The straight chain alkanes share the same general formula:
\[c_{n}h_{2n+2}\] the general formula means that the number of hydrogen atoms.
Therefore, alkanes are refered to as a homologous series. Alkynes contain four hydrogen atoms less than corresponding alkanes and two hydrogen atoms less than corresponding alkenes and have the general formula c n h 2 n − 2. The longest continuous chain should include both the carbon atoms of the triple bond. First member is ethyne c 2 h 2.
Names start in “cyclo” (cyclopentane, cyclooctane, etc.) 7.
The hybridization of an alkane is sp3 while that of alkenes is sp2 and that of alkynes is sp. C x n h 2 n + 2. In organic chemistry, an alkane or paraffin is an acyclic saturated hydrocarbon. The general molecular formula of alkanes is {eq}c_nh_2n+2 {/eq}, where n is an integer number that represents the number of atoms in a compound.
C x n h 2 n + 2.
Alkanes are saturated as they are linked only by single bonds. Alkanes formula and its condensed structure; Saturated hydrocarbons have only single bonds and are rather unreactive. Table boiling points of alkenes and alkynes name formula boiling point (°c) ethylene ch2 d;ch2 −103.7 acetylene hc≡ch −84.0 propene ch2 d;chch3 −47.6 propyne hc≡cch3 −23.2 1 butene ch2 d;chch2ch3 −6.1 cis 2 butene cis ch3ch d;chch3 b;3.7
The alkanes are also called as paraffins.
The typical isomeries of alkanes are the structure (or chain) isomerism and the conformational isomerism. Identify each as an alkane, an alkene, or an alkyne. The general formula of alkenes is c n h 2n. Alkanes have the general formula c n h 2n+2.
Example of an alkene eg:
This gives them a general formula : List of alkanes and its structures; Any alkane with 18 or more carbon atoms are solid. Reaction in which halogen is introduced into a molecule 3) hydration :
Formula for cyclic alkanes c nh 2n
The name olefin is derived from the greek word olefin gas, which means oil forming. Contains a carbon‐carbon double bond and has the general formula cnh2n. Unsaturated hydrocarbons have double or triple bonds and are quite reactive; Alkenes undergo self addition in which alkene molecules join together to form long chains called polymers.
Each alkane is different from previous by one carbon and two hydrogens.

![Alkane Formula [with free study guide]](https://i2.wp.com/www.aceorganicchem.com/blog/wp-content/uploads/2017/08/Alkanes.png)