Reaction in which halogen is introduced into a molecule 3) hydration : The alkanes are also called as paraffins. The next few members are liquids and the higher ones are solids.
Solved Draw The Structure Of The Alkene That Would Give O
Alkanes have the general formula c n h 2n+2 and can be subdivided into the following three groups:
Alkenes having four or more carbon atoms can form diverse structural isomers.most alkenes are also isomers of cycloalkanes.acyclic alkene structural isomers with only one double bond follow:
Ethene ( c 2 h 4), propene (c 3 h 6) etc. For example, an alkane with 2(n) carbon atoms, will have 6 (2n+2) hydrogen atoms. (i) alkanes have general formula c n h 2 n + 2 hence for 20 carbon atoms formula will be c 2 0 h 4 2 (ii) alkenes have general formula c n h 2 n → c 2 0 h 4 0 (iii) alkynes have general formula c n h 2 n − 2 → c 2 0 h 3 8. Methane gas is the first member of the homologous series of alkanes.
1) addition of hydrogen halides 2) halogenation :
Alkene alkyne four major additions: Alkanes have the general formula c n h 2n+2. In an alkane, all 4 4 4 valencies of the carbon atom are satisfied with other hydrogen atoms. Except for ethylene, all other alkenes are colourless and odourless.
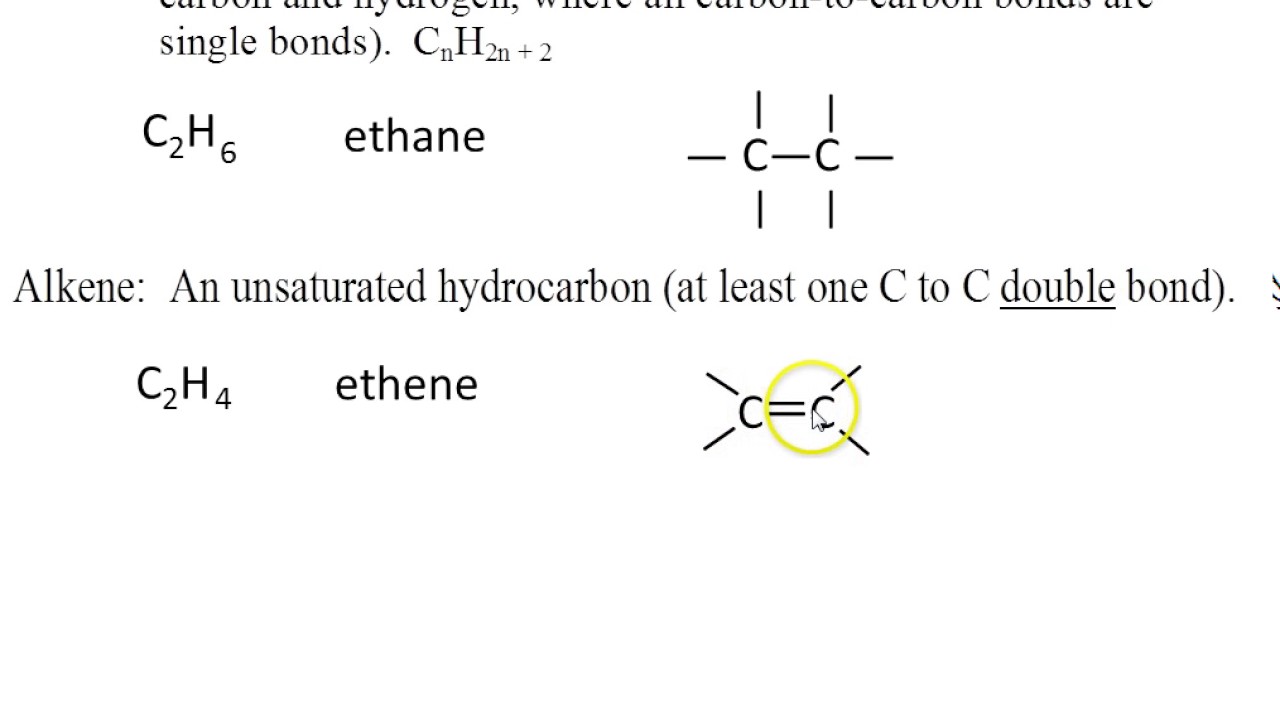
Alkenes are unsaturated hydrocarbons having a double bond between the carbon atoms.
The general formula of alkenes is c n h 2n. A fully saturated hydrocarbon, an alkane, has general formula cnh 2n+2: The name olefin is derived from the greek word olefin gas, which means oil forming. Reaction in which the elements of water (h and oh) are
The general molecular formula of alkanes is {eq}c_nh_2n+2 {/eq}, where n is an integer number that represents the number of atoms in a compound.
“alkanes & alkenes in chemistry” is an interactive app for students to learn about the alkanes, alkenes, reactions of alkenes, alkane to alkene, organic chemistry, alkene formula, alkynes in an easy and engrossing way by visualizing the colorful images. These three kinds of alkanes are straight chain alkanes, branched chain alkanes and cycloalkanes. Please log in or register to add a comment. General formula for alkyl radical is c n h 2 n + 1 hence option a is correct.
First member is methane (ch 4) also known as “marsh gas”, chief constituent of natural gas (~97%) hybridisation sp 3.
C x n h 2 n + 2. What is the general formula of alkanes and cycloalkanes? Alkane is (organic chemistry) any of saturated hydrocarbones including methane, ethane and compounds with long carbon chain known as paraffins etc, having a chemical formula of the form cnh2n+2 while alkyle is (organic chemistry) any of series of univalent radicals of the general formula cnh2n+1. Ethylene is a colourless gas with a sickly sweet odour.
For example in the diagram, the four hydrocarbon molecules contain 8 carbon atoms each.
C x n h 2 n + 2. Alkanes have the general formula of c n h 2n + 2 where n is the number of carbon atoms. General formula for alkane, alkene and alkyne are c n h 2 n + 2 , c n h 2 n , and c n h 2 n − 2. Alkanes can be obtained by hydrogenation of alkenes by means of hydrogen and catalyst.
Their general formula (not including cyclic compounds) is c n h 2n.
The alkyl group is made from an alkane (a hydrocarbon without multiple bonds) through the removal or loss of a hydrogen atom. Methane (ch 4) ethane (c 2 h 6) etc. C n h 2n •cycloalkane name will usually be the base compound •number carbons in ring if >1 substituent •first in alphabet gets lowest number if more than one substituent •in case where a long chain is attached to cycloalkane then give the name of the chain with cyclic alkane as cycloalkyl group. The general formula is c n h 2n+2.
The general structural formula of alkynes is rccr', where r and r' correspond to alkyl side chains, and the cc corresponds to two carbon atoms that are joined by a triple.
Alkenes are often used as a synonym of olefin. 2.3 reactions of alkenes and alkynes ⇒ additions are the most common reactions using alkenes and alkynes addition to: (1997)] against carbon number is shown in figure 2. Alkenes have the general formula c n h 2n.
(1982a, 1995a) and this work] and cyclohexane [data from aschmann et al.
The general formula of the homologous series of alkanes is cnh2n + 2 where n is an integer. Alkenes resemble alkanes in most of their physical properties. Is that alkane is (organic chemistry) any of saturated hydrocarbons including methane, ethane and compounds with long carbon chain known as paraffins etc, having a chemical formula of the form c n h 2n+2 while alkyl is (organic chemistry) any of a series of univalent radicals of the general formula c n h 2n+1 derived from aliphatic hydrocarbons.