Alkaline earth metal is smaller in size when compared to the corresponding alkali metals. The salts are colourless unless they include a coloured anion (negative ion). The chemical reactivity of alkaline earth metals increases on moving down the group from be to ba, because the ionization enthalpy decreases and the electrode potential.
Chemical Properties of Alkali Metals YouTube
All alkaline earth metals burn in n 2 and form ionic nitrids m 3 n 2.
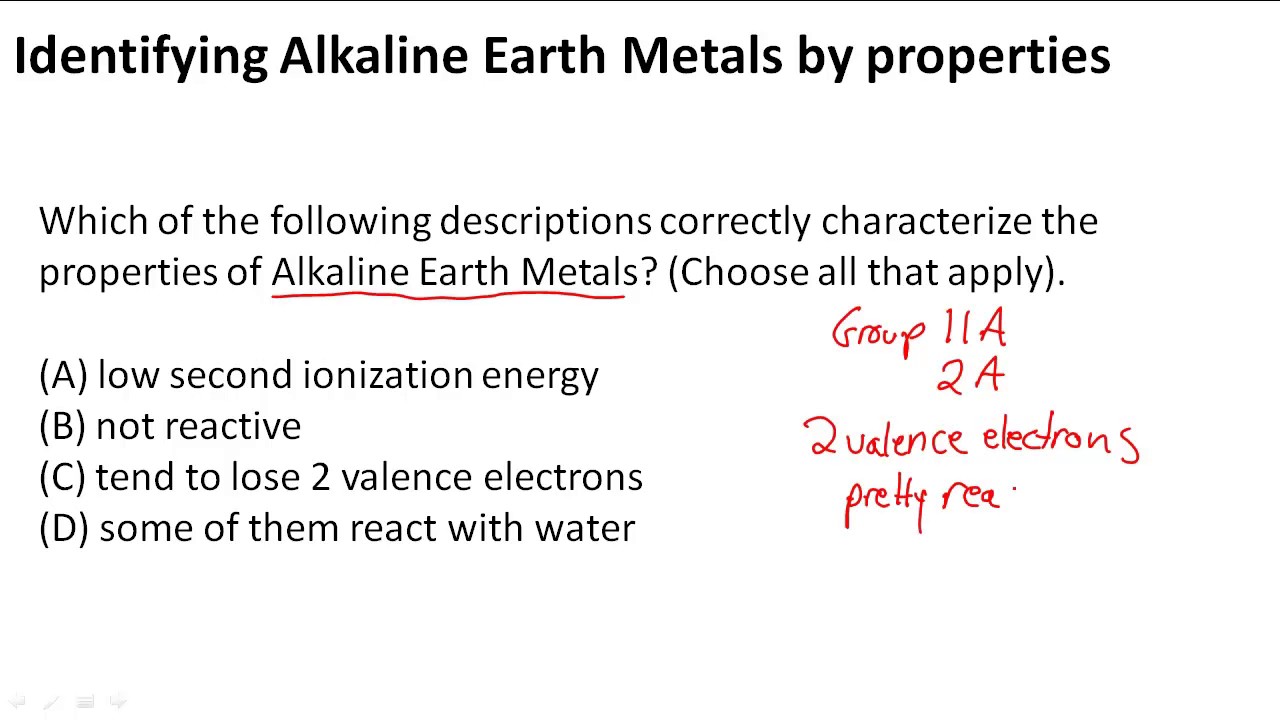
Alkaline earth metals (group 2) majorly includes six elements namely beryllium (be), magnesium (mg), calcium (ca), strontium (sr), barium (ba), and radium (ra).
10 rows the alkaline earth metals are the elements that correspond to group 2 of the modern periodic. The alkaline earth metals are six chemical elements in group 2 of the periodic table.they are beryllium (be), magnesium (mg), calcium (ca), strontium (sr), barium (ba), and radium (ra). They possess low melting and boiling points. Be 3 n 2 is rather volatile.
Somewhat reactive metals at standard temperature and pressure
All the nitrides are all crystalline solids which. Properties of alkaline earth metals. ( that’s why the word “earth” is used) Alkali metals have only +1 ionic charge in their compounds when alkaline earth metals have +2 ionic charges in their compounds.
Moreover, alkali metals are very soft and they can be cut with a sharp knife.
Alkaline earth metals uniformly show an oxidation state of +2. They are generally softer and less dense than transition metals. They all have significantly higher melting points than the alkali metals. Therefore, they can easily lose these two electrons to form divalent cation.
At standard temperature and pressure, the elements have extremely similar properties:
The bond in alkaline earth metal compounds is mostly ionic in character. The large amount of energy required comes from the very large amount of lattice energy evolved when th e crystalline solid is formed. The alkaline earth metals are a set of six chemical elements in the periodic table’s group 2. This group includes beryllium (be), magnesium (mg), calcium (ca), strontium (sr), barium (ba) and radium (ra).
Engineering and manufacturing density functional theory density functionals electric waves electromagnetic radiation electromagnetic waves electromagnetism solar energy
N 2 molecule is very stbale. The reactivity of alkaline earth metals is high however, it is relatively less when compared to the reactivity of alkali metals. Beryllium (be), magnesium (mg), calcium (ca), strontium (sr), barium (ba), and radium (ra) are the elements involved (ra). Salts in which the metal occurs as the cation m 2+, where m represents any group 2 atom.
Their oxides exist in earths’s crust and are very stable to heat.
The alkaline earth metal ions always have an oxidation number of +2 and their compounds are mainly stable, colorless and most of them are solid ionic substance. This high reactivity is due to low ionization energy and high negative values of standard electrode potential. Being in the second group, all of the alkaline earth elements have 2 electrons in their outer electron shell, in the s orbital. Alkaline earth metal prefer to form divalent ions rather than monovalent ions
Properties of alkaline earth metals.
But magnesium and beryllium compounds show that the covalent character in their bond is greater than ionic character. Comparatively, alkali metals are more reactive than alkaline earth metals. And all alkaline earth metals have two outer electrons. By advances in materials science and engineering;
These metals forms +2 ions only.
The general electron configuration of alkaline metals is [noble gas] ns 2 where n represents the valence shell. The alkaline earth metals are those that make up group 2 of the periodic table, and are indicated in the purple column in the image below. Barium (alkaline earth metal) lantanum (lanthanide) serium (lanthanide) praseodimium (lanthanide) neodimium (lanthanide) prometium (lanthanide) samarium (lanthanide) europium. (i) reactivity towards air and water.
The alkaline earth metals have two electrons more than the nearest noble gas configuration.
Alkaline earth metals reactions, uses, properties. Most of their typical compounds are therefore ionic: This group usually contain smooth, silver metals with a less metallic character than the elements of group 1. However, they have higher cohesive energy, higher ionization potential and higher enthalpy of sublimation as well.
Beryllium and magnesium are kinetically inert to oxygen and water due to an oxide film on their surface.
Chemical properties of alkaline earth metal. The elements have very similar properties: Alkaline earth metals are difficult to find in their pure form in nature. Powdered beryllium burns brilliantly on ignition in air to give beo and be3n2.
From top to bottom, they are beryllium, magnesium, calcium, strontium, barium, and radium.
Structurally, they (together with helium) have in. Magnesium is more electropositive and burns with dazzling brilliance in air to give mgo and mg3n2. An excellent mnemonic method to remember their names is through the pronunciation of mr.




.PNG)