For example, dilute nitric acid oxidizes both the aldehyde group and. Ketoses are monosaccharides that contain a ketone group per molecule. What is the difference between ketose and aldose?
Solved PartA Classify Each Of The Following As An Aldose
Examples of ketone are fructose, ribulose and xylulose, erythrulose, tagatose, sorbose, pentoses, hexoses, heptoses, octoses, nonoses, tetroses etc.
Ketose is an impure sugar.
A ketose is a sugar with one ketone group for every molecule. An example of an aldose: 8 rows ketose sugars like fructose dehydrate faster than aldose sugars like glucose. Aldose is a pure sugar.
On the other hand, ketose gets defined as a monosaccharide that has a ketone group in each molecule that contains three carbon atoms.
On the contrary, the examples of ketose sugar are fructose, erythrulose, and ribulose. Aldose contains an aldehyde (cho) group (consisting of carbon, hydrogen, and oxygen), while ketose contains a ketone (co) group (consisting of carbon and oxygen). A ketose is a monosaccharide consisting of a carbon backbone and a carbonyl group within the backbone. • ketoses are monosaccharides with a ketone group.
Aldoses are primarily found in plants.
Only in the presence of reducing sugar, they can isomerize to aldose. A good example is fructose. With such type of sugars ketoses can isomerize into an aldose when carbonyl group is present at the last of the molecule. Aldose is a pure sugar.
The ketoses which react quickly will produce a dark red colour.
Aldoses tend to isomerise into ketoses. In ketoses, carbonyl carbon has number two. Ketoses can be found in processed foods. Aldoses are primarily found in plants.
Ketoses can be found in processed foods.
Aldoses react in a slow manner resulting in a light pink colour whereas; An aldose is a monosaccharide (a simple sugar) with a carbon backbone chain with a carbonyl group on the endmost carbon atom, making it an aldehyde, and hydroxyl groups connected to all the other carbon atoms. Ketoses can isomerise into aldoses inly if the carbonyl group is at the end of the chain. Ketose is a monosaccharide whose carbon skeleton has a ketone group.
A great way to remember this difference is to focus on the first letter in each term:
Both aldoses and ketoses are reducing sugars.stronger oxidizing agents can oxidize other hydroxyl groups of aldoses. A good example is glucose. The anomeric carbon in cyclic aldoses has a hydrogen opposite the hydroxyl group, while cyclic ketoses have a hxdroxylmethyl in that position. An example of ketose is fructose.
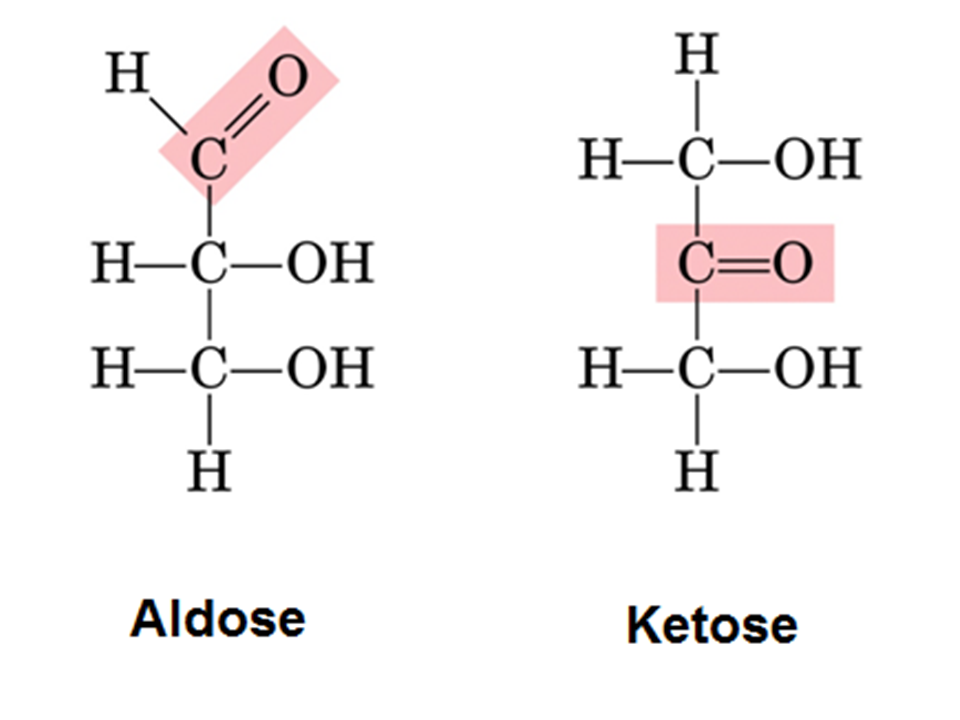
Aldoses are monosaccharides with an aldehyde group.
Aldose contains an aldehyde group, and ketose contains a ketone group. Aldose is a monosaccharide with an aldehyde group. Difference between aldose and ketose definition. If the carbonyl group of a sugar is an aldehyde, the sugar is called an aldose.
Ketoses have ketone as the functional group.
Aldose is the monosaccharide that contains aldehyde group in its structure along with the carbon chain, whereas ketose is the monosaccharide that contains ketone group along with the carbon chain. Ketose is an impure sugar. An example of aldose is glucose. Aldoses have aldehyde as the functional group.
If the carbonyl group of a sugar is a ketone, the sugar is called a ketose.
An aldose is a monosaccharide (a simple sugar) with a carbon backbone chain with a carbonyl group on the endmost carbon atom, making it an aldehyde, and hydroxyl groups connected to all the other carbon atoms. Examples of aldose are glycolaldehyde, glyceraldehydes, erythrose, threose, glucose and galactose. They are used in processed food. The functional groups in each sugar are different.
From what we have learned, can you identify the main difference between aldose and ketose sugars?
• ketoses form hemiketal rings and aldoses form hemiacetal rings. An aldose sugar contains an aldehyde functional group in its structure; Aldose sugars that contain more than three carbon atoms possess. Examples of ketose are ribulose, fructose, etc.
Ketose sugars contain ketone functional groups.
Aldoses can be distinguished from ketoses, which have the carbonyl group away from the end of the molecule, and are therefore ketones. Aldoses can be distinguished from ketoses, which have the carbonyl group away from the end of the molecule, and are therefore. An aldose is defined as a monosaccharide whose carbon skeleton has an aldehyde group. Examples of aldose sugar are glucose, galactose, and ribose.
Aldose gets defined as the monosaccharide that only has one aldehyde group in each molecule and becomes a pure sugar.
Aldose and ketose are both simple sugars ( monosaccharides ). Differences between aldose and ketose sugars. The chemical names of the ketose sugars depend on the number of carbon atoms they possess. They are primarily found in plants.
• in aldoses, the carbonyl group is in the number one position.
Glucose and ribose are aldoses. The mechanism of the reaction is mainly based on dehydration reaction which occurs quicker in the ketoses than the aldoses. Aldoses are monosaccharides that contain an aldehyde group per molecule.