View aldose and ketose.docx from che organic ch at mariano marcos state university. Those monosaccharides that contain an aldehyde functional group are called aldoses; In this nomenclature, a group of up to four consecutive chiral carbons is named after the aldose (triose, tetrose, pentose, or hexose) possessing this chiral group, and the number.
Pin on school
Ketoses can isomerise into aldoses inly if the carbonyl group is at the end of the chain.
The one equivalent of reagent is utilized to oxidize the hydroxyl group to the carbonyl group.
Ketose and aldose are monosaccharides which can be differentiated based on the group they contain. This article is cited by 20 publications. Carbohydrates are the main sources of energy in the body. Aldoses vs ketoses epimeric pair (summarizing epimers) to summarize;
6 rows ketose and aldose are monosaccharides which can be differentiated based on the group they.
Aldose (c=o) is located at one end of the carbohydrate sugarforming an aldehyde group.ketose (c=o) is located at the internal position of thecarbohydrate sugar forming a ketone group. Is sucrose a reducing sugar? Aldose structure has one carbon atom. Molecules having one actual or potential sugar group are called monosaccharides.
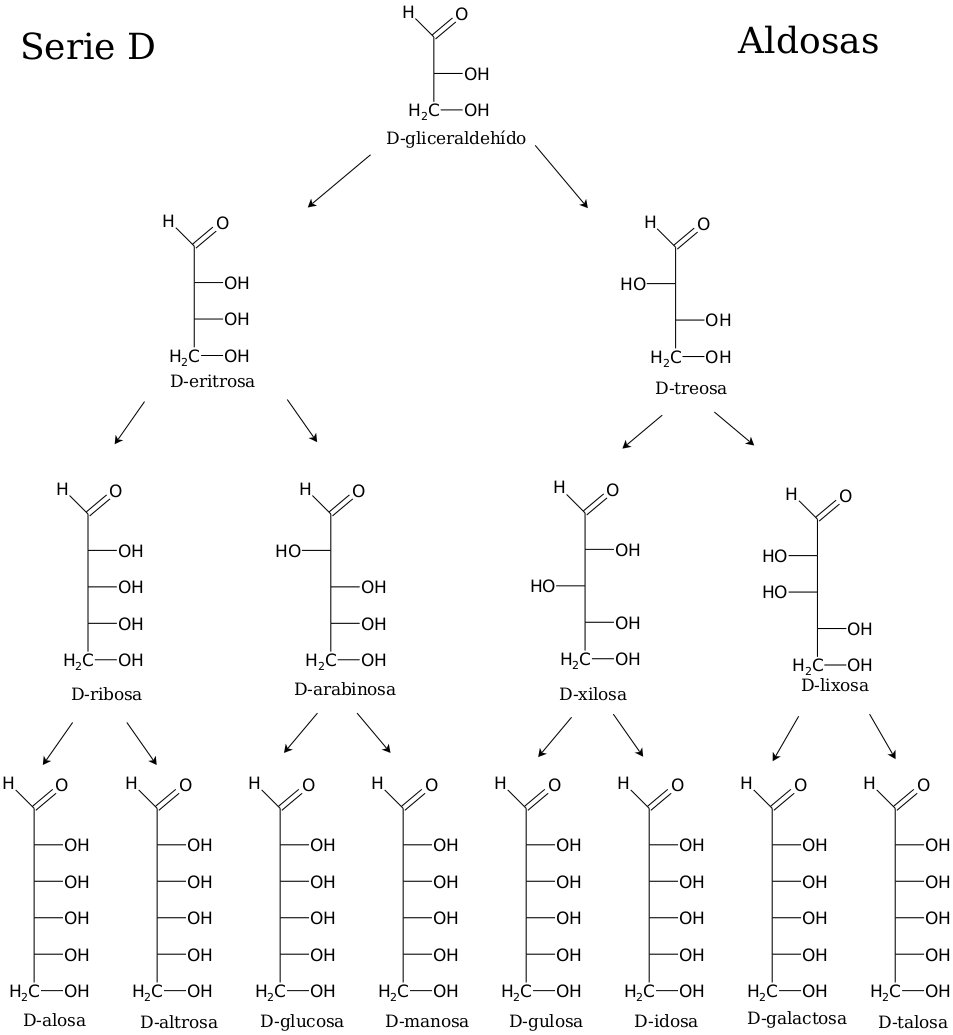
Distinguishing common monosaccharides from each other on the basis of structural characteristics
Aldose are aldehyde groups whereas ketose belongs to the ketone group. What is the most common aldose ketose? Ketose (c=o) is located at the internal position of the carbohydrate sugar forming a ketone group. Combining these classification systems gives general names that indicate both the type of carbonyl group and the number of carbon atoms in a molecule.
Aldoses are monosaccharides that contain an aldehyde group per molecule.
Epimers are a special class of diastereoisomers. If the sugar contains an aldehyde bunch, it is an aldose. Those containing a ketone functional group on the second carbon atom are ketoses. Difference between aldose and ketose definition.
Aldose (c=o) is located at one end of the carbohydrate sugar forming an aldehyde group.
If we compare the osazone formation of glucose (aldose) and fructose (ketose) we observe that both utilized the three equivalents of the reagent but the product contains the two phenyl hydrazine residues. Aldose is the monosaccharide that contains aldehyde group in its structure along with the carbon chain. In which of the following pairs of monosaccharides is one member of the pair an aldose and the other a ketose? Examples of ketose are ribulose, fructose, etc.
Ketoses are monosaccharides that contain a ketone group per molecule.
Aldoses tend to isomerise into ketoses. Sucrose, or table sugar, is a disaccharide consisting of both fructose and glucose. Although glucose is an aldose, several ketoses like fructose also have epimers i.e. Aldose is found in plants generally but ketones are found in processed food.
Match the carbohydrates on the left with their aldose/ ketose pair carbohydrate on the right.
Greek word mono means one and saccharide means sugar. Ketose is the monosaccharide that contains ketone group along with the carbon chain. An aldose is defined as a monosaccharide whose carbon skeleton has an aldehyde group. Number of carbon atoms :
What are aldose and ketose?