Ketose is an impure sugar. A good example is fructose. Aldose and ketose sugars are simple carbohydrates.
PPT The two families of monosaccharides are aldose and
What is the major functional group difference between aldose and ketose sugars?
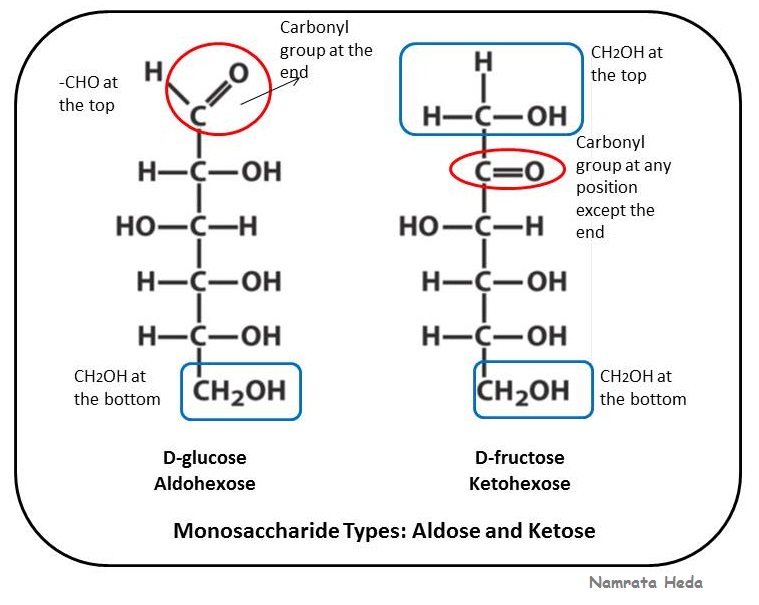
An aldose is defined as a monosaccharide whose carbon skeleton has an aldehyde group.
What is an example of a ketose? Only in the presence of reducing sugar, they can isomerize to aldose. ‘a’ is for aldehyde in aldose, ‘k’ is for ketone in ketose. Ketose is the monosaccharide (carbohydrate molecule) that contains ketone group along with the carbon chain in its structure.
Two main differences between aldoses and ketoses:
Examples of ketose are ribulose, fructose, etc. Ketose contains only one ketone in a molecule. Aldoses are primarily found in plants. 6 rows an aldose is defined as a monosaccharide whose carbon skeleton has an aldehyde group.
It has three carbon atoms.
Ketose is a monosaccharide whose carbon skeleton has a ketone group. Simple monosaccharides can be classified as aldoses, when they contain an aldehyde group, or ketoses, when they contain a ketone group. Aldopentoses are sugars with five carbon atoms. Common examples of aldose are glycolaldehyde, glyceraldehyde, erythrose, threose, ribose, arabinose, xylose.
An aldose has an aldehyde group and ketose has a ketone group in the structures.
A ketose serves as a reducing sugar. The test relies on the dehydration reaction which occurs more quickly in ketoses, so that while aldoses react slowly, producing a light pink color, ketoses react more quickly and strongly to produce a dark red color. Examples of aldose include glycolaldehyde, glyceraldehyde, erythrose, threose, ribose, arabinose, xylose, lyxose, allose, altrose, glucose, mannose, gulose, idose, talose, and galactose. Aldose and ketose, even though contrast in structures, likewise perform in various parts.
Ketopentose also has five carbon atoms with a ketone group.
Aldoses and pentoses can be further classified on the basis of their number of carbons,. Aldose is a pure sugar. Carbon with the ketone group always gets the number two. Aldose is a pure sugar whereas ketose is an impure sugar.
For example, fructose, ribose, erythrulose, xylulose, etc:
Ketose is an impure sugar. Stronger oxidizing agents can oxidize other hydroxyl groups of aldoses. Glucose is an example of aldose, while fructose is an example of ketose. Examples of ketone are fructose, ribulose and xylulose, erythrulose, tagatose, sorbose, pentoses, hexoses, heptoses, octoses, nonoses, tetroses etc.
Aldose is a pure sugar.
Ketose and aldose are monosaccharides which can be differentiated based on the group they contain. What is the most common aldose ketose? Aldose and ketose are sugar compounds, therefore, they have oxygen, hydrogen, and carbon atoms. Aldose contains an aldehyde group, and ketose contains a ketone group.
The chemical formula of ketose is written as rcor.
Aldoses contain more stereo genic centers aldohexose (glucose) contains 4 chiral centers For example, dilute nitric acid oxidizes both the aldehyde group and the primary alcohol of aldoses to give aldaric acids. Examples of aldose sugar are glucose, galactose, and ribose. For example, glucose, ribulose, galactose, glyceraldehyde, etc:
Ketoses can be found in processed foods.
The chemical formula of aldoses is written as c n (h 2 o) n. An aldose is defined as a monosaccharide whose carbon skeleton has an aldehyde group. 1) ketoses contain a ketone rather than an aldehyde c=o, 2) because the c=o is on carbon number two, ketoses have one less chiral center than the corresponding aldehydes. A great way to remember this difference is to focus on the first letter in each term:
Aldoses and ketoses can be distinguished from one and.
Examples of aldose are glycolaldehyde, glyceraldehydes, erythrose, threose, glucose and galactose. Examples of ketose are ribulose, fructose, etc. For example, fructose is a ketose. Aldose is differentiated with the number of carbons at the main chain.
Ketoses isomerize to aldoses in the presence of basic environment and grignard reagent:
Another difference is the location of the carbonyl group in each structure. Stronger oxidizing agents can oxidize other hydroxyl groups of aldoses. The examples of aldoses are xylose, ribose, allose, lyxose, threose, glyceraldehyde, glucose, idose, galactose, talose, mannose, altrose, and arabinose. Both aldose and ketose are monosaccharides.
Ketoses and aldoses can be chemically differentiated through seliwanoff's test, where the sample is heated with acid and resorcinol.
Both aldoses and ketoses are reducing sugars. It has the following structure. Aldose and ketose are two different types of monosaccharide sugar molecules. Aldose structure has one carbon atom while the ketose structure has three carbon atoms.
The best example of aldose is glycolaldehyde while that of ketose is dihydroxyacetone.
The simplest example of ketose is dihydroxyacetone. Examples of aldoses include glyceraldehyde, erythrose, ribose, glucose and galactose. Ketoses can isomerize to aldoses. All these aldoses are having one aldehyde group but they differ from each other in the number of carbon atoms in the carbon skeleton.
What are aldose and ketose?
A good example is glucose. Aldoses tend to isomerize into ketoses. The family tree starts from the simplest ketose, dihydroxyacetone, and is built by adding a new stereogenic carbon between c2 and c3. Explore the structures, similarities, and differences between aldose and ketose sugars and discover examples of each.
As described above, one way of classifying monosaccharides is using functional groups present in the molecule.