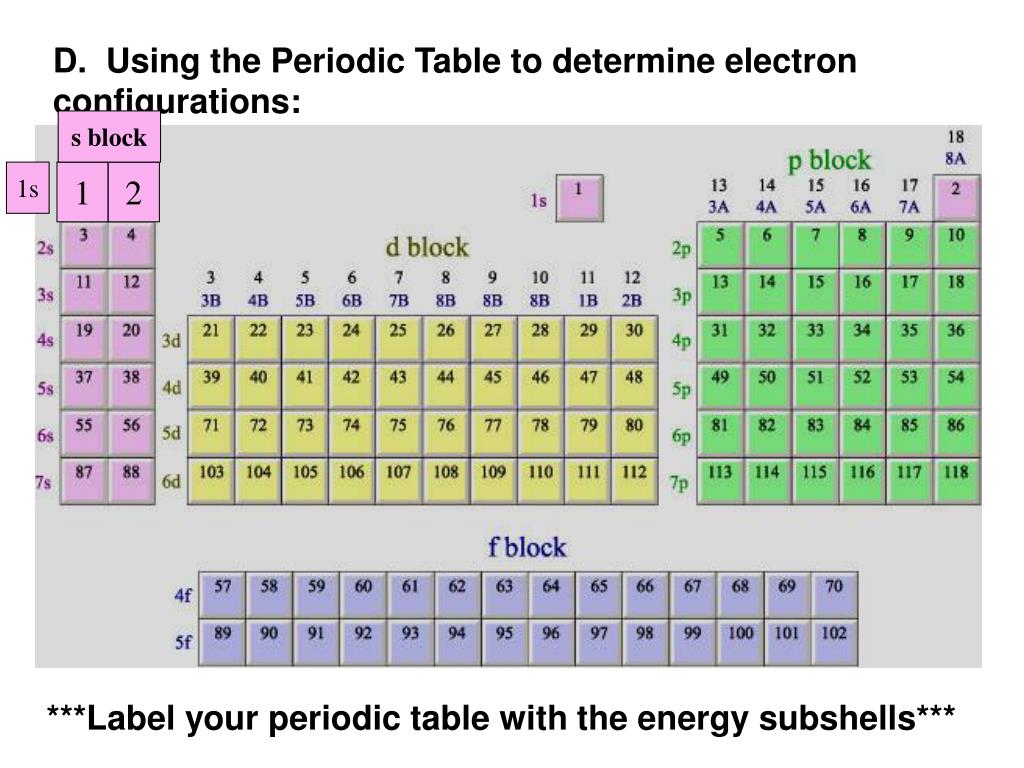
The s block on the periodic table are groups 1 and 2; The electronic configuration of each element is decided by the aufbau principle which states that. 2s 2 p 2 :
Daily Quiddity Positive Quiddity The Periodic Table
1s 2 2s 2 p 2 :
Electron configurations, filling orbitals, and valence electrons of 2p elements.
A chemical reaction does not occur for this question. The bracketed noble gas symbols on the left represent the inner configurations that are the same in each period. The electron configuration of boron is: The two colors show the phase or sign of the wave function in each region.
Each picture is domain coloring of a ψ (x, y, z) function which depend on the coordinates of one electron.
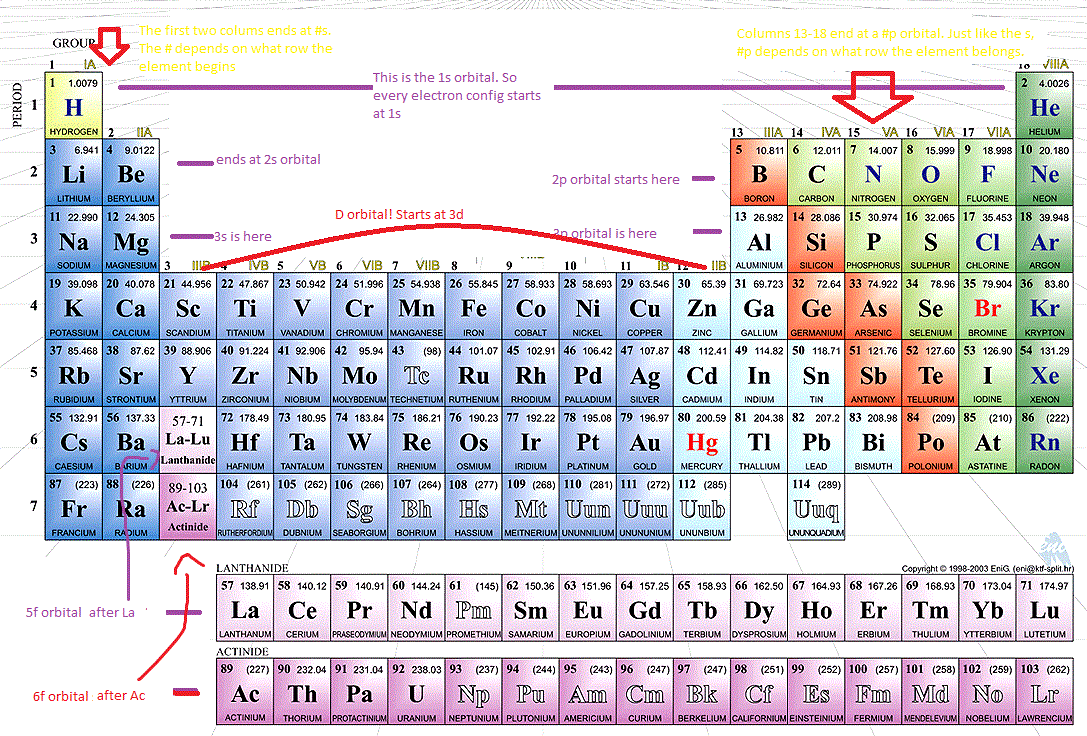
Gen atom, the order of increasing orbital energy is given by 1s < 2s = 2p < 3s = 3p = 3d, etc. The periodic table a simple version with just a symbol. Atomic radii trend how does an atom’s position on the periodic table relate to its atomic radius? There is a simple way of remembering how electrons fill up orbitals, shown in the accompanying diagrams:
Neon with atomic number of 10 has an electron configuration of 1s 2 2s 2 2p 6.
2s 2 p 1 : 2p elements in periodic table. Part a number of c atoms in 0.439 mol of c ve ασφ ε ? 30 rows we see that the atomic shells fill up in the order 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d,.
N=4 n=5 n=3 n=2 m l l l l l n n.
Boron (atomic number 5) has five electrons. Periodic table of the elements. D and f block example : 1s < 2s = 2p
Here, carbon has four unpaired electrons.
Hydrogen has one electron which represents the lowest energy level. For example, (he2s 2p would be entered as [he]2s^22p^2. Electrons will occupy different orbitals in a given subshell, before two electrons will occupy a single orbital. Atomic numbers 3 and 4 are in the second row of the s block (look for them in the bottom half of in image below), signifying that the 3rd and 4th electrons are in the 2s sublevel.
Part b 1s 2s 2p 3 sº3p4s 3d express your answer as a chemical symbol.
Similarly, 3s, 3p and 3d increase energy in that order, and so on. The shapes of the first five atomic orbitals are: Four electrons fill both the 1s and 2s orbitals. The f block on the periodic table are the lanthanide and actinide series.
1s 2s 3s 4s 5s 6s 7s 2p 3p 4p 5p 6p 7p 3d 4d 5d 6d 7d 4f 5f 6f 7f.
Use the periodic table to give the symbol of the element with each of the following electron configurations: They end in s1 and s2. The electronic configuration of each and every element in the periodic table reflects its character, stability and their chemical as well as physical properties. Applying the diagonal rule • simply count electrons until the sublevel is filled, and then move to the next sublevel in the order given by the diagonal.
1s 2 2s 2 p 1 :
Also, the valency of an element is determined by electron configuration in the excited state. Submit request answer calculate each of the following: Neon is one of the noble gases because its outer orbitals have enough electrons to be full. 1s, 2s, 2p x, 2p y, and 2p z.
Table 5.2 shows the electron configurations of the elements with atomic numbers 1 through 18.
It is given that 1s 2p that means 1s, 2s orbitals are filled and then 2p orbitals are maximum filled, then it contains 9 electrons, that is 2 electrons in 1s orbital, then 2 electrons in 2s orbital then 5 electrons in the. 1s 2 2s 2 2p 1. Atomic numbers 5 through 10 are in the first row of the p block, and the p sublevels start on the second energy level. Thus 2s lies below 2p, as already observed in helium.
Submit request answer part b silver express your answer in abbreviated form, in order of increasing orbital energy.
The order of filling is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s. Periodic table vrije universiteit amsterdam. Let’s look at some examples. Therefore, the electron configuration of carbon(c*) in excited state will be 1s 2 2s 1 2p x 1 2p y 1 2p z 1.
S and p blocks 1s, 2s, 2p 1s, 2s, 2p, 3s, 3p, 3d (available) 17 periodic table:
6d 1s 2s 3s 4s 5s 6s 2p 3p 4p 5p. Grayed out electron numbers indicate subshells that are filled to their maximum. We know that the first two electrons added to an atom go to the 1s sublevel. When the carbon atom is excited, then the carbon atom absorbs energy.
< 14 of 22 (> review constants periodic table part a iron express your answer in abbreviated form, in order of increasing orbital energy.
1s 2 2s 2 p 3 :